What is the role of integrin clustering in focal adhesion assembly?
There is still much debate over the mechanisms of signal transduction following integrin-ligand binding, however recent studies reveal that irrespective of the global density of integrins, local clustering of ligand-bound integrins is paramount to efficient signal transduction [1]. The minimum cluster area required for stable FA assembly and force transmission has a dynamic nanolimit, regulated by the interplay between adhesive force, cytoskeletal tension and the structural linkage that transmits them [2]. These initial clusters (specifically αVβ3 integrins) serves as a platform for the tethering and polymerization of actin filaments [3][4].
From available data, the following course of events is hypothesized to be the working model for FA initiation (reviewed in [5], see figure below). Actin–talin–integrin complexes stabilize both focal adhesions and stress fibers through the recruitment of additional components such as focal adhesion kinase (FAK) [6][7], paxillin [8][9] and Src-family kinases (SFKs) to integrin tails [10][11]. The phosphatase, RPTP-α, is known to activate SFKs and in doing so reinforce αVβ3-cytoskeletal connections [12][13]. While talin can further enhance PIP2 production and FAK activation [14][15], FAK has been shown to reinforce clusters by promoting talin recruitment [16]. Src-dependent actin polymerization initially pushes the clusters outwards.
Subsequently, myosin II contraction brings adjacent actin-linked clusters closer to one another and inwards [4]. Actomyosin contractility has also been shown to stretch talin [17], revealing binding sites for other proteins, such as vinculin [18][19][20]. This has been shown to occur in living cells, with an integrin-talin-vinculin-actin link being established in a stretch-dependent manner. Here, stretching of the cell substrate resulted in vinculin accumulation at the focal adhesion site. This link was sufficient to arrest the retrograde flow of actin filaments, and promote the formation of cellular protrusions [21]. Thus it is believed that cycles of talin deformation, vinculin binding and release by the slipping actin filaments [22] integrate the traction exerted on the substrate to biochemical signals [17].
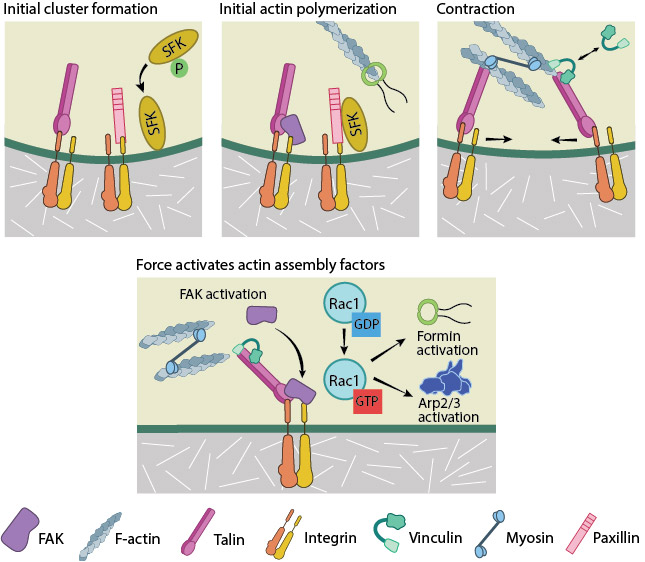
Adapted from [32]. Initial integrin clusters (top left), after activation by talin binding, provide avenue for initial actin polymerization (top middle) by recruiting focal adhesion components- FAK, SFKs and paxillin. New actin filaments tether to talin, the clusters get pushed away and then pulled closer by myosin contractions (top right). This causes cycles of transient talin stretching and vinculin binding until the talin- actin bond stabilizes. Upon stable vinculin binding (bottom), further integrin clustering and signaling promote Rac1 activation. Rac1, in turn, further activates actin polymerization modules, Arp2/3 and formins.
Additionally, the link between the actin cytoskeleton and integrins can also be further stabilized by the recruitment of the IPP cytosolic ternary complex [31], comprising integrin-linked kinase (ILK), parvin and PINCH (particularly interesting Cys-His-rich protein). Integrin-related kinase (ILK) [32] is able to bind to the separated tails of activated β1 and β3 integrins [33][34]. The IPP complex is recruited to focal adhesions to promote cytoskeleton linkage and integrin signaling [34][35] through several binding partners including paxillin and kindlin (reviewed in [36]).
References
- Schvartzman M, Palma M, Sable J, Abramson J, Hu X, Sheetz MP, and Wind SJ. Nanolithographic control of the spatial organization of cellular adhesion receptors at the single-molecule level. Nano Lett. 2011; 11(3):1306-12. [PMID: 21319842]
- Coyer SR, Singh A, Dumbauld DW, Calderwood DA, Craig SW, Delamarche E, and García AJ. Nanopatterning reveals an ECM area threshold for focal adhesion assembly and force transmission that is regulated by integrin activation and cytoskeleton tension. J. Cell. Sci. 2012; 125(Pt 21):5110-23. [PMID: 22899715]
- Butler B, Gao C, Mersich AT, and Blystone SD. Purified integrin adhesion complexes exhibit actin-polymerization activity. Curr. Biol. 2006; 16(3):242-51. [PMID: 16461277]
- Yu C, Law JBK, Suryana M, Low HY, and Sheetz MP. Early integrin binding to Arg-Gly-Asp peptide activates actin polymerization and contractile movement that stimulates outward translocation. Proc. Natl. Acad. Sci. U.S.A. 2011; 108(51):20585-90. [PMID: 22139375]
- Roca-Cusachs P, Iskratsch T, and Sheetz MP. Finding the weakest link: exploring integrin-mediated mechanical molecular pathways. J. Cell. Sci. 2012; 125(Pt 13):3025-38. [PMID: 22797926]
- Ren XD, Kiosses WB, Sieg DJ, Otey CA, Schlaepfer DD, and Schwartz MA. Focal adhesion kinase suppresses Rho activity to promote focal adhesion turnover. J. Cell. Sci. 2000; 113 ( Pt 20):3673-8. [PMID: 11017882]
- Shi Q, and Boettiger D. A novel mode for integrin-mediated signaling: tethering is required for phosphorylation of FAK Y397. Mol. Biol. Cell 2003; 14(10):4306-15. [PMID: 12960434]
- Brown MC, Perrotta JA, and Turner CE. Identification of LIM3 as the principal determinant of paxillin focal adhesion localization and characterization of a novel motif on paxillin directing vinculin and focal adhesion kinase binding. J. Cell Biol. 1996; 135(4):1109-23. [PMID: 8922390]
- Mofrad MRK, Golji J, Abdul Rahim NA, and Kamm RD. Force-induced unfolding of the focal adhesion targeting domain and the influence of paxillin binding. Mech Chem Biosyst 2004; 1(4):253-65. [PMID: 16783922]
- Arias-Salgado EG, Lizano S, Sarkar S, Brugge JS, Ginsberg MH, and Shattil SJ. Src kinase activation by direct interaction with the integrin beta cytoplasmic domain. Proc. Natl. Acad. Sci. U.S.A. 2003; 100(23):13298-302. [PMID: 14593208]
- Reddy KB, Smith DM, and Plow EF. Analysis of Fyn function in hemostasis and alphaIIbbeta3-integrin signaling. J. Cell. Sci. 2008; 121(Pt 10):1641-8. [PMID: 18430780]
- Jiang G, Huang AH, Cai Y, Tanase M, and Sheetz MP. Rigidity sensing at the leading edge through alphavbeta3 integrins and RPTPalpha. Biophys. J. 2005; 90(5):1804-9. [PMID: 16339875]
- von Wichert G, Jiang G, Kostic A, De Vos K, Sap J, and Sheetz MP. RPTP-alpha acts as a transducer of mechanical force on alphav/beta3-integrin-cytoskeleton linkages. J. Cell Biol. 2003; 161(1):143-53. [PMID: 12682088]
- Ling K, Doughman RL, Firestone AJ, Bunce MW, and Anderson RA. Type I gamma phosphatidylinositol phosphate kinase targets and regulates focal adhesions. Nature 2002; 420(6911):89-93. [PMID: 12422220]
- Kong X, Wang X, Misra S, and Qin J. Structural basis for the phosphorylation-regulated focal adhesion targeting of type Igamma phosphatidylinositol phosphate kinase (PIPKIgamma) by talin. J. Mol. Biol. 2006; 359(1):47-54. [PMID: 16616931]
- Lawson C, Lim S, Uryu S, Chen XL, Calderwood DA, and Schlaepfer DD. FAK promotes recruitment of talin to nascent adhesions to control cell motility. J. Cell Biol. 2012; 196(2):223-32. [PMID: 22270917]
- Margadant F, Chew LL, Hu X, Yu H, Bate N, Zhang X, and Sheetz M. Mechanotransduction in vivo by repeated talin stretch-relaxation events depends upon vinculin. PLoS Biol. 2011; 9(12):e1001223. [PMID: 22205879]
- Lee SE, Kamm RD, and Mofrad MRK. Force-induced activation of talin and its possible role in focal adhesion mechanotransduction. J Biomech 2007; 40(9):2096-106. [PMID: 17544431]
- Ziegler WH, Gingras AR, Critchley DR, and Emsley J. Integrin connections to the cytoskeleton through talin and vinculin. Biochem. Soc. Trans. 2008; 36(Pt 2):235-9. [PMID: 18363566]
- del Rio A, Perez-Jimenez R, Liu R, Roca-Cusachs P, Fernandez JM, and Sheetz MP. Stretching single talin rod molecules activates vinculin binding. Science 2009; 323(5914):638-41. [PMID: 19179532]
- Hirata H, Tatsumi H, Lim CT, and Sokabe M. Force-dependent vinculin binding to talin in live cells: a crucial step in anchoring the actin cytoskeleton to focal adhesions. Am. J. Physiol., Cell Physiol. 2014; 306(6):C607-20. [PMID: 24452377]
- Jiang G, Giannone G, Critchley DR, Fukumoto E, and Sheetz MP. Two-piconewton slip bond between fibronectin and the cytoskeleton depends on talin. Nature 2003; 424(6946):334-7. [PMID: 12867986]
- Humphries JD, Wang P, Streuli C, Geiger B, Humphries MJ, and Ballestrem C. Vinculin controls focal adhesion formation by direct interactions with talin and actin. J. Cell Biol. 2007; 179(5):1043-57. [PMID: 18056416]
- Puklin-Faucher E, and Sheetz MP. The mechanical integrin cycle. J. Cell. Sci. 2009; 122(Pt 2):179-86. [PMID: 19118210]
- Bos JL. Linking Rap to cell adhesion. Curr. Opin. Cell Biol. 2005; 17(2):123-8. [PMID: 15780587]
- Brindle NP, Holt MR, Davies JE, Price CJ, and Critchley DR. The focal-adhesion vasodilator-stimulated phosphoprotein (VASP) binds to the proline-rich domain in vinculin. Biochem. J. 1996; 318 ( Pt 3):753-7. [PMID: 8836115]
- Reinhard M, Halbrügge M, Scheer U, Wiegand C, Jockusch BM, and Walter U. The 46/50 kDa phosphoprotein VASP purified from human platelets is a novel protein associated with actin filaments and focal contacts. EMBO J. 1992; 11(6):2063-70. [PMID: 1318192]
- DeMali KA, Barlow CA, and Burridge K. Recruitment of the Arp2/3 complex to vinculin: coupling membrane protrusion to matrix adhesion. J. Cell Biol. 2002; 159(5):881-91. [PMID: 12473693]
- Serrels B, Serrels A, Brunton VG, Holt M, McLean GW, Gray CH, Jones GE, and Frame MC. Focal adhesion kinase controls actin assembly via a FERM-mediated interaction with the Arp2/3 complex. Nat. Cell Biol. 2007; 9(9):1046-56. [PMID: 17721515]
- Le Clainche C, and Carlier M. Regulation of actin assembly associated with protrusion and adhesion in cell migration. Physiol. Rev. 2008; 88(2):489-513. [PMID: 18391171]
- Zhang Y, Chen K, Tu Y, Velyvis A, Yang Y, Qin J, and Wu C. Assembly of the PINCH-ILK-CH-ILKBP complex precedes and is essential for localization of each component to cell-matrix adhesion sites. J. Cell. Sci. 2002; 115(Pt 24):4777-86. [PMID: 12432066]
- Li F, Zhang Y, and Wu C. Integrin-linked kinase is localized to cell-matrix focal adhesions but not cell-cell adhesion sites and the focal adhesion localization of integrin-linked kinase is regulated by the PINCH-binding ANK repeats. J. Cell. Sci. 1999; 112 ( Pt 24):4589-99. [PMID: 10574708]
- Hannigan GE, Leung-Hagesteijn C, Fitz-Gibbon L, Coppolino MG, Radeva G, Filmus J, Bell JC, and Dedhar S. Regulation of cell adhesion and anchorage-dependent growth by a new beta 1-integrin-linked protein kinase. Nature 1996; 379(6560):91-6. [PMID: 8538749]
- Pasquet J, Noury M, and Nurden AT. Evidence that the platelet integrin alphaIIb beta3 is regulated by the integrin-linked kinase, ILK, in a PI3-kinase dependent pathway. Thromb. Haemost. 2002; 88(1):115-22. [PMID: 12152651]
- Tucker KL, Sage T, Stevens JM, Jordan PA, Jones S, Barrett NE, St-Arnaud R, Frampton J, Dedhar S, and Gibbins JM. A dual role for integrin-linked kinase in platelets: regulating integrin function and alpha-granule secretion. Blood 2008; 112(12):4523-31. [PMID: 18772455]
- Legate KR, Montañez E, Kudlacek O, and Fässler R. ILK, PINCH and parvin: the tIPP of integrin signalling. Nat. Rev. Mol. Cell Biol. 2006; 7(1):20-31. [PMID: 16493410]


