
Shailaja SEETHARAMAN
Assistant Professor, Mechanobiology Institute, National University of Singapore
shailaja@nus.edu.sg
10-03G
Level 10 T-Lab
National University of Singapore
5A Engineering Drive 1
Singapore 117411
Shailaja Seetharaman
Principal Investigator
Research Areas
Endothelial cell dynamics, Vascular mechano-medicine pathways
Research Interests
Endothelial cells are the thin, specialized cells that line the interior surface of all blood and lymphatic vessels. They form a continuous, dynamic interface between circulating blood and the vessel wall, and their health underpins the function of nearly every organ. As a semi-permeable layer, endothelial cells regulate vascular tone, maintain blood fluidity and clotting, orchestrate immune cell trafficking, and drive angiogenesis and repair. Endothelial dysfunction is a key driver of major diseases including atherosclerosis, hypertension and cancer.
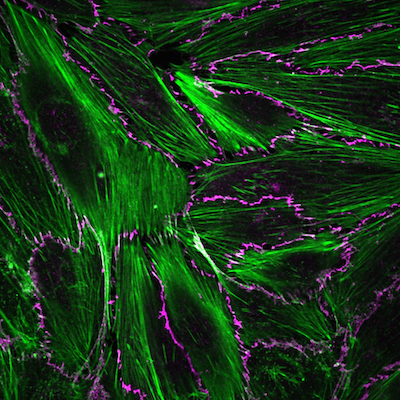 Our vision is to decode, predict, and engineer disease-relevant mechano-responses of the endothelium. The biological questions that motivate us include: how does the endothelium sense and respond to mechanical forces, including fluid shear stress, stretch, and substrate curvature? How does mechanosensing mediate cardio- and cerebrovascular function, and how does mechanosensing go awry to drive diseases? We will provide crucial mechanistic insight into how long timescale, force-dependent gene expression drives rapid biochemical signaling and phenotypical behaviors to ultimately tune vascular tissue function across scales. Decoding these precise mechano-chemical feedback loops will help establish models to tune tissue function and bridge the gap between the fields of biology and medicine.
Our vision is to decode, predict, and engineer disease-relevant mechano-responses of the endothelium. The biological questions that motivate us include: how does the endothelium sense and respond to mechanical forces, including fluid shear stress, stretch, and substrate curvature? How does mechanosensing mediate cardio- and cerebrovascular function, and how does mechanosensing go awry to drive diseases? We will provide crucial mechanistic insight into how long timescale, force-dependent gene expression drives rapid biochemical signaling and phenotypical behaviors to ultimately tune vascular tissue function across scales. Decoding these precise mechano-chemical feedback loops will help establish models to tune tissue function and bridge the gap between the fields of biology and medicine.
Our lab focuses on two fundamental themes incorporating both experimental and modeling approaches: 1) decoding the precise mechanobiology of vascular function in cardio- and cerebro-vascular diseases, and 2) domain-tailored predictive modeling and control of tissue mechanoresponses in disease. The overarching theme is to map how transcriptional status and local cellular events precisely define tissue behavior and function across scales. Using in vitro approaches as well as patient heart tissues, and leveraging advanced high-resolution microscopy, multi-omics approaches, biochemistry, and bioengineering tools, we will decode the mechano-chemical feedback loops driving vascular tissue dysfunction and establish models to tune them. This will allow us to engineer adaptive tissues to tune physiology and enable targeted interventions in diseases where biological systems go awry.
Education
MS in Biomedical and Molecular Sciences, King’s College London, UK
PhD in Cell and Developmental Biology, Institute Pasteur, Paris, France
Biography
Shailaja Seetharaman is leading the vascular mechano-medicine lab at MBI, with a joint appointment as an Assistant Professor in the Department of Physiology, NUS School of Medicine. Prior to this, she was a postdoctoral fellow in the Department of Physics at the University of Chicago. She received her PhD in Cell and Developmental Biology from Institut Pasteur, Paris, and her Master’s in Biomedical and Molecular Sciences from King’s College London. Her work has been supported by the American Heart Association, Eric and Wendy Schmidt AI in Science, and Yen Postdoctoral Fellowships, and the Marie Curie and the Fondation pour la Recherche Médicale PhD Fellowships.
Shailaja is looking to recruit graduate students to join her lab. Please contact her via email or visit her website for more details.
Recent Publications
- Seetharaman S*, Devany J, Kim HR, van Bodegraven E, Chmiel T, Tzu-Pin S, Chou WH, Fang Y, Gardel ML*. Mechanosensitive FHL2 tunes endothelial function. bioRxiv. 2024 Jun 17; PubMed Central PMCID: PMC11212908. *co-corresponding author.
- Schmitt MS, Colen J, Sala S, Devany J, Seetharaman S, Caillier A, Gardel ML, Oakes PW, Vitelli V. Machine learning interpretable models of cell mechanics from protein images. Cell. 2024 Jan 18;187(2):481-494.e24. PubMed PMID: 38194965.
- van Bodegraven E, Pereira D, Peglion F, Infante E, Kesenci Y, Terriac E, Geay J, Roca V, Plays M, Soto L, Seetharaman S, Boëda B, Olivo-Marin JC, Asnacios A, Boquet-Pujadas A, Manneville JB, Etienne-Manneville S. Intermediate filaments promote glioblastoma cell invasion by controlling cell deformability and mechanosensitive gene expression. Research Square [Preprint]. 2023 June 29. Available from: https://doi.org/10.21203/rs.3.rs-2828066/v1
- Geay J*, Seetharaman S*, Vianay B, Gélin M, Fresnoy O, Blanchoin L, Théry M. Deletion of FAT1 in hybrid EMT cells stimulates the migration of neighboring non-mutant cells through secretion of extracellular vesicles. bioRxiv [Preprint]. 2023 September 6. *co-first author.
- Seetharaman S*, Sala S*, Gardel ML, Oakes PW. Quantifying Strain-Sensing Protein Recruitment During Stress Fiber Repair. Methods Mol Biol. 2023;2600:169-182. PubMed PMID: 36587097. *co-first author.
- Deb Roy A, Gross EG, Pillai GS, Seetharaman S, Etienne-Manneville S, Inoue T. Non-catalytic allostery in α-TAT1 by a phospho-switch drives dynamic microtubule acetylation. J Cell Biol. 2022 Nov 7;221(11) PubMed Central PMCID: PMC9565784.
- Seetharaman S, Vianay B, Roca V, Farrugia AJ, De Pascalis C, Boëda B, Dingli F, Loew D, Vassilopoulos S, Bershadsky A, Théry M, Etienne-Manneville S. Microtubules tune mechanosensitive cell responses. Nat Mater. 2022 Mar;21(3):366-377. PubMed PMID: 34663953.
- Seetharaman S, Etienne-Manneville S. Cytoskeletal Crosstalk in Cell Migration. Trends Cell Biol. 2020 Sep;30(9):720-735. PubMed PMID: 32674938.
- Seetharaman S, Etienne-Manneville S. Microtubules at focal adhesions – a double-edged sword. J Cell Sci. 2019 Oct 9;132(19) PubMed PMID: 31597743.
- Bance B*, Seetharaman S*, Leduc C, Boëda B, Etienne-Manneville S. Microtubule acetylation but not detyrosination promotes focal adhesion dynamics and astrocyte migration. J Cell Sci. 2019 Apr 5;132(7) PubMed PMID: 30858195. *co-first author.
- De Pascalis C, Pérez-González C, Seetharaman S, Boëda B, Vianay B, Burute M, Leduc C, Borghi N, Trepat X, Etienne-Manneville S. Intermediate filaments control collective migration by restricting traction forces and sustaining cell-cell contacts. J Cell Biol. 2018 Sep 3;217(9):3031-3044. PubMed Central PMCID: PMC6122997.
- Seetharaman S, Etienne-Manneville S. Integrin diversity brings specificity in mechanotransduction. Biol Cell. 2018 Mar;110(3):49-64. PubMed PMID: 29388220.
- Seetharaman S, Flemyng E, Shen J, Conte MR, Ridley AJ. The RNA-binding protein LARP4 regulates cancer cell migration and invasion. Cytoskeleton (Hoboken). 2016 Nov;73(11):680-690. PubMed Central PMCID: PMC5111583.
Lab Members
Brandon Goh Yeow Wee
Research Fellow, Seetharaman Group
Hana Maldivita Tambrin
Research Fellow, Seetharaman Group




