The Mechanism of Osmosensing by EnvZ and other HKs
Mapping conformational changes in EnvZ associated with osmotic stress
We used amide hydrogen/deuterium exchange mass spectrometry in collaboration with Dr. Ganesh Anand in the Department of Biological Sciences at NUS to map conformational changes in EnvZ associated with osmotic stress. We discovered that the osmosensing apparatus is localized to a four-helix bundle subdomain (Wang et al., 2012). Specifically, a helical segment containing the EnvZ auto-phosphorylation site (His243) exhibits decreased exchange in response to increasing osmolality (NaCl, sucrose), while an adjacent helical peptide exists in multiple conformations in solution. ATP binding to the kinase subdomain is independent and uncoupled from osmosensing.
Surprisingly, the inner membrane protein EnvZ does not need to be in the membrane for osmosensing. Our results support a model where osmolytes promote intrahelical H-bonding that enhances helix stabilization and increase the rate of autophosphorylation (Fig. 2). This model combines coupling of osmolyte-induced helix stabilization with downstream signaling and likely provides a conserved mechanism for signaling proteins that respond to diverse physical and mechanical stimuli. Further tests of the model are underway in our laboratory.
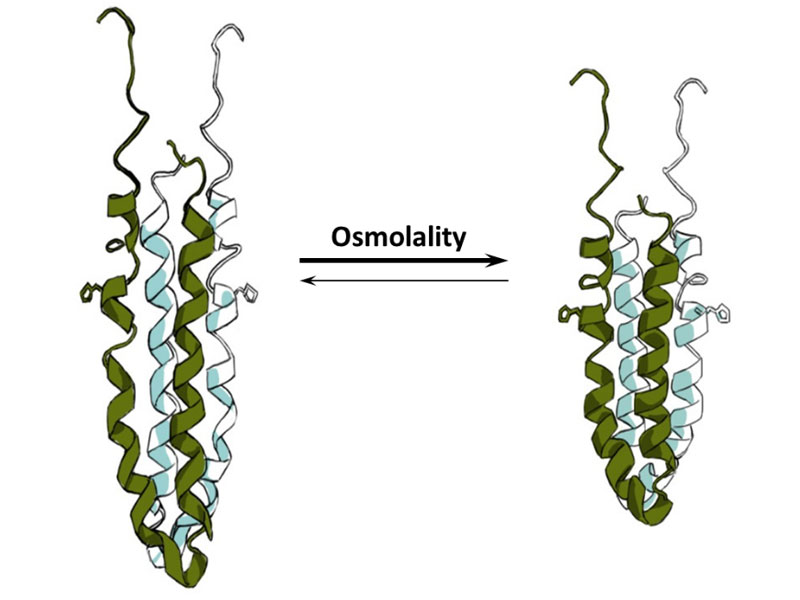
Coil to helix transcription is the mechanism of osmosensing by the EnvZ histidine kinase. At low osmolality, the histidine-containing helix (shown with the His side-chain sticking out) is slightly unfolded and histidine autophosphorylation is low. At high osmolality, this peptide shows increased hydrogen bonding leading to helix stabilization and increased autophosphorylation.
Researchers: Jeremy Wang, Soumya Ranganathan
Lab: Linda J Kenney Lab


