The mechanical editor
How Myosin II proofreading tightens the heart
Written by Sruthi Jagannathan | Schematic provided by the Saunders Lab | October 2020
During development, precise matching between early embryonic cells is critical for the various organs to form correctly. In this study, Dr. Shaobo Zhang and MBI Principal Investigator Associate Professor Timothy Saunders at describe how periodic filopodial retraction caused by the contractile activity of transient Myosin II clusters at the cell leading edge ensures precise matching between primitive heart cells during heart development in Drosophila embryos.
An artistic depiction of Drosophila heart cells putting forth filopodia to look for and connect with the right partner cell during heart formation.
Myosin II-dependent filopodial retraction ensures precise matching of heart cells in Drosophila embryos
Just as we appreciate the value of making the right connections in life, cells in a developing embryo have an innate propensity to make the right ‘cellular connections’ as they form new tissues and organs. The precision of the cellular matchmaking process enables vital organs in our body, including the brain and heart, to assemble robustly and develop without defects.
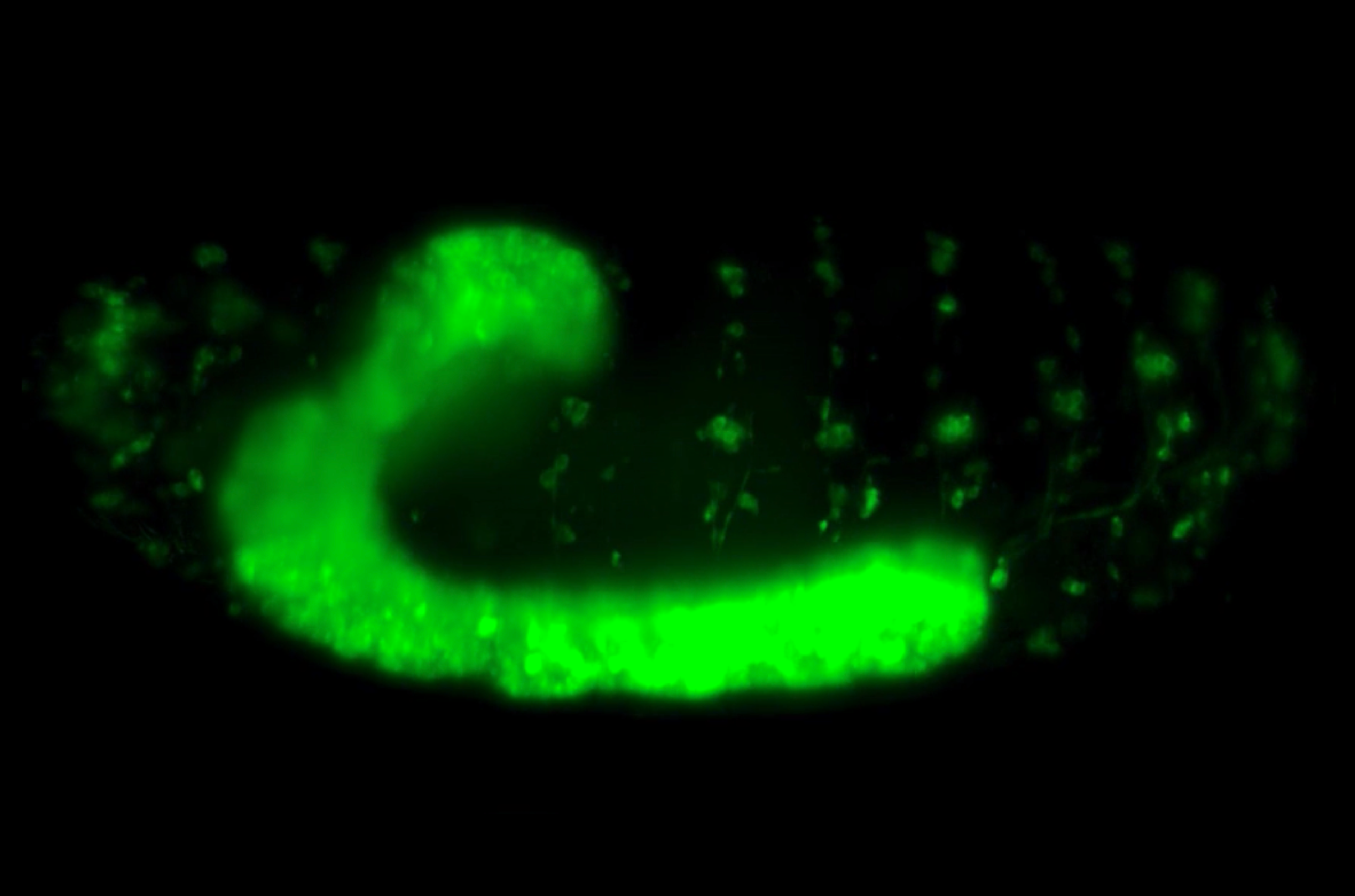
Earlier work from the lab of Principal Investigator Associate Professor Timothy Saunders, investigated how heart development in Drosophila is driven by the formation of precise physical connections between primitive heart cells in a developing embryo. During early stages of heart development, two opposing rows of cells align along the midline and cells on one row physically connect with partner cells on the other side.
The Saunders lab revealed how these developing heart cells extend protrusions called filopodia to probe for partner cells. There are specific adhesion molecules present on filopodial surfaces, which help in establishing stable connections with the right partner. These connections effectively ‘button-up’ the heart, sealing the midline and giving rise to rudimentary heart tubes.
By demonstrating how Myosin II-mediated contractility at the leading edge facilitates periodic backward pulling of filopodia, this study highlights the importance of such an additional mechanical proofreading mechanism in ensuring precise cell matching during Drosophila heart formation.
Building upon these findings, the latest research from the Saunders lab, led by Dr. Shaobo Zhang and published in Current Biology, has now identified another molecular player – the Myosin II motor – in the matchmaking process. Along with actin, the contractile activity of the actomyosin network generates forces to carry out fundamental cellular processes.
Periodic filopodial retraction driven by Myosin II contractility ensures precise heart cell matching
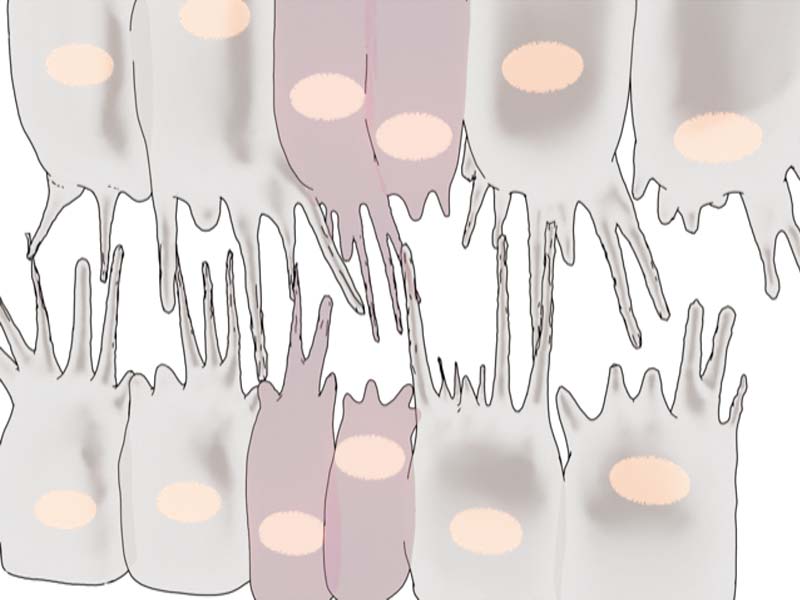
By adopting a combination of techniques – genetic perturbations to block Myosin II activity, laser ablation to cut filopodia, and live imaging to view heart formation in Drosophila embryos – the researchers found that Myosin II forms transient clusters at the leading edges of heart cells that retract into the cytoplasm soon after. When the clusters are at the leading edge, they generate contractile forces on the filopodia, that results in backward pulling or retraction of the filopodia. When the clusters are away from the leading edge, the filopodia are able to extend and make connections with opposing filopodia.
The researchers suggest that filopodial retraction mechanically facilitates precise matchmaking by providing two levels of regulations. Firstly, random connections initiated loosely with incorrect partner cells are pulled apart before they can stabilize further. Secondly, connections initiated between the right partners are further reinforced and stabilized by the periodic backward pulling – similar to how a loose knot is tightened by the pulling of the strings.
This mechanical proofreading mechanism acts as a quality control process, in which Myosin II contractile activity checks the precision of cellular matchmaking during heart formation. Consistent with this, Myosin II depleted embryos showed defects in heart cell matching. Defective cell matching during embryogenesis could have serious consequences in adult life forms. For instance, pairing up of incorrect cell partners during heart formation causes incomplete closure of the heart, which results in life threatening congenital heart defects post birth.
By shedding more light on the molecular and mechanical mechanisms that control the robustness of heart development, this study enhances the scope for scientists and clinicians to better understand such disorders and devise clinical interventions.