Taking pole positions
How polarity proteins localize at opposite poles during symmetry breaking
Written by Sruthi Jagannathan | Illustration by MELANIE LEE | September 2018
Symmetry breaking is an important event during cell polarization that is initiated by the non-uniform distribution of molecules across the cell. A recent study led by Assistant Professor Fumio Motegi, Principal Investigator at the Mechanobiology Institute and the Temasek Lifesciences Laboratory, National University of Singapore, has revealed the simplest molecular network that mediates the distribution of polarity regulators at opposite ends of the cell during symmetry breaking. The study, published in Nature Chemical Biology, has been featured as the cover image for the journal.

An artistic depiction of how PAR-2 herds PAR-1 to a safe region and protects it from PKC-3 activity.
A minimal network that establishes opposing cortical gradients during polarization
Cell polarity is the inherent asymmetry seen in cells- in their structure, chemical composition, or cellular functions. The polarization of cells is an essential prerequisite that allows several fundamental cellular processes to take place. For instance, division of epithelial cells relies on the apical-basal polarization of cells so that each daughter cell inherits distinct top and bottom surfaces. Similarly, the movement of cells during tissue organization in embryos or during the life of an adult organism requires the polarization of cells into front and rear ends.

This study, published in Nature Chemical Biology, was featured as the cover image for the October 2018 issue of the journal.
The establishment of cell polarity occurs as early as the start of life: soon after a zygote (fertilized cell) is formed and before the zygote starts dividing into the first set of founder cells. It sets a global pattern for polarization for all other newly forming embryonic cells as well as in cells formed in the adult organism. Therefore, understanding how polarity is established in zygotes will shed light on the basic cellular and molecular mechanisms that take place during this fundamental biological phenomenon.
How is symmetry breaking initiated?
Symmetry breaking is an important step in cell polarization, during which an initially symmetrical zygote becomes asymmetrical in response to polarity-inducing signals. As part of this symmetry breaking process, certain groups of proteins move in a targeted manner to opposite poles of the zygote. The polarity regulators in each pole, in turn, associate with and recruit other cellular components, and together they contribute to the distinctive structures and functions of that cellular region.
In order to understand the symmetry breaking process in detail, it is important to know what causes the polarity regulators to move in a targeted manner to different regions of the cell.
With this question in mind, a team of researchers from the National University of Singapore, led by Assistant Professor Fumio Motegi, set out to investigate the dynamic interactions that take place between polarity regulators and whether these interactions cause the regulators to be localized in distinct regions during the earliest stages of development.
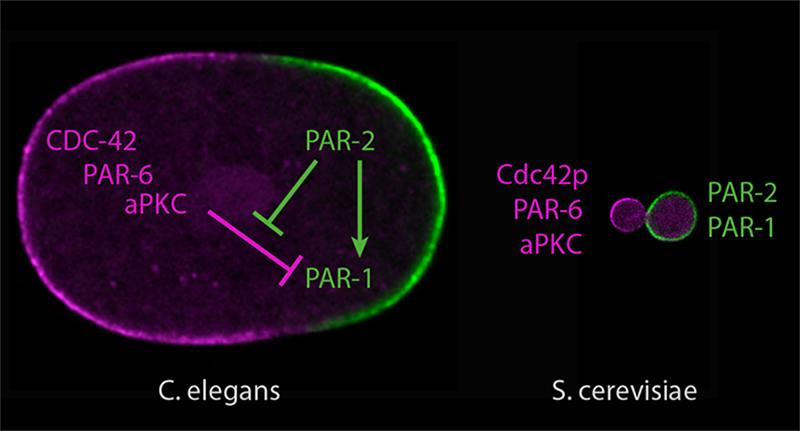
The image shows the opposing distribution of PKC-3 (in purple) and PAR-1 (in green) at the anterior and posterior poles of C. elegans zygote., respectively. PAR-2 plays a role in positioning PAR-1 at the posterior pole and shielding it from exclusion by PKC-3.
Conducting their studies in C. elegans (roundworm) zygotes, the research team focused on the localization patterns of two important polarity regulators- PKC-3 and PAR-1. These two protein kinases are known to localize to opposing ends of the zygote during polarization. PKC-3 is distributed at the future head (anterior) region of the zygote, and PAR-1 is located at the future tail (posterior) region.
To determine how PKC-3 and PAR-1 take up opposite positions in the cell, the researchers introduced synthesized fragments of PAR1 and PKC3 that were tagged with fluorescent dyes into C. elegans zygotes and tracked the localization patterns of these regulators by imaging whole zygotes using confocal microscopy.
Based on their observations, the researchers proposed that the opposite localization patterns of polarity regulators is dependent on a ‘mutual inhibition’ mechanism, whereby both proteins function to exclude each other from their vicinity. Further, they revealed that this mutual inhibition requires an important role for another regulator, PAR-2, in mediating this mutual inhibition mechanism.
By tracking the localization patterns of polarity regulators through confocal microscopes, Motegi and team describe a minimal molecular network that is able to initiate symmetry breaking during polarization of C. elegans zygotes.
PAR-2, which is mainly present at the posterior pole of the zygote, physically associates with PAR-1 to position it at this end. This interaction shields PAR-1 from exclusion by PKC-3. Additionally, PAR-2 also stabilizes PAR-1 at the posterior pole, by slowing its diffusive movement. At the other side, PKC-3 localization at the anterior pole is driven by a combination of mechanical flows arising inside the cell that transport PKC-3, and the exclusion by PAR-2-PAR-1 at the posterior end.
Based on their observations, the researchers concluded that PAR-1, PAR-2, and PKC-3 serves as the simplest molecular network that is able to establish opposing localization patterns of polarity regulators during the symmetry breaking process. They further tested if this simplest molecular network could be sufficient to induce symmetry breaking by adopting a synthetic biology approach. Remarkably, replicating this network by introducing synthetic constructs of PAR-1, PAR-2, and PKC-3 into fungal S. cerevisiae cells led to the establishment of opposing localization patterns of these polarity regulators, demonstrating that this animal cell polarity network can be reconstituted in non-animal cells.
The study successfully defines the simplest molecular network that is essential for breaking symmetry during cell polarization. The discovery of this basic building block for polarity provides vital insights into understanding the basic molecular events that trigger the earliest life processes in a multicellular organism. Moreover, the synthetic biology approach described here can be applied to synthesize not just polarity units but also other functional molecular units, which can be out to multiple physiological or clinical uses in the lab: from engineering tissues or organs for regenerative medicine to reprogramming cellular or organism behavior for targeted therapies.