Guiding random events to create resilient patterns
The Mechanobiology of Development lab at the Mechanobiology Institute, National University of Singapore and University of Warwick, UK uncovers how random cell fusion events during muscle development are regulated at the tissue level to ensure correct patterning of the muscle architecture. This study was published in Developmental Cell, https://doi.org/10.1016/j.devcel.2022.08.002.
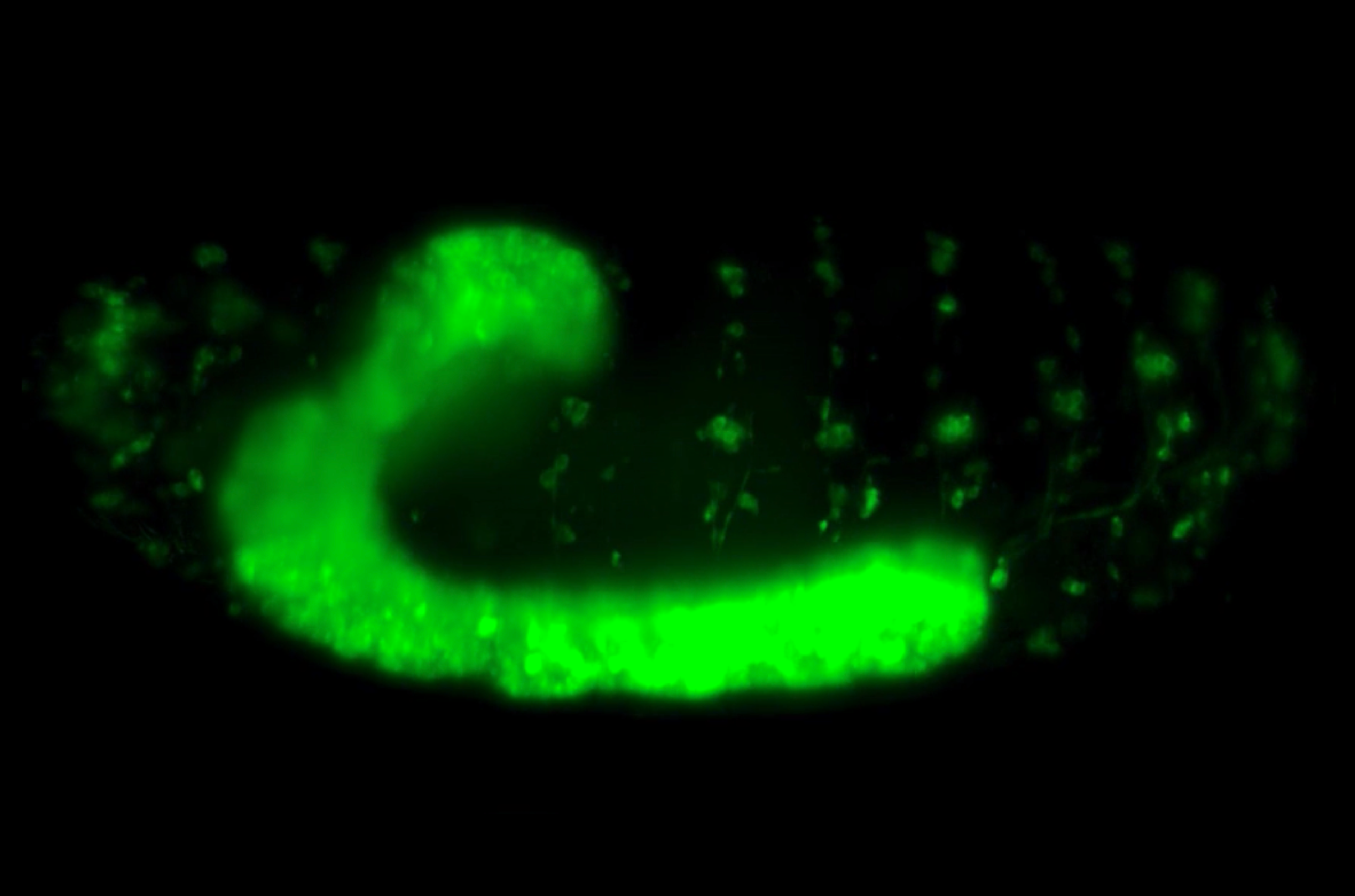
Skeletal muscle is made up of two types of muscle fibres, slow-twitch and fast-twitch. It is known that slow-twitch fibres are required for endurance exercise such as long-distance running, while fast-twitch fibres are used for explosive movements such as sprinting. Both slow and fast muscle fibres originate from the same muscle progenitor cells, which either elongate to form slow muscle cells, or fuse with other muscle progenitor cells to generate multinucleated fast muscle fibres in the case of the fast muscle cells. Despite all this knowledge of muscle cell development at the cellular level, an unanswered question remained as to how muscle tissue arranges into a characteristic striated, or striped pattern of muscle fibres (Figure 1).
Figure 1: Striated pattern of muscle fibres in Zebrafish embryo.
This lack of understanding was partly due to technical limitations. Scientists often had to rely on snapshots of muscle tissue development and infer from these images the potential changes that had occurred. Think of a city planner trying to understand traffic flow during rush hour with just static photos for a scale of the challenge. In both cases, live imaging, either of muscle cell movement or traffic flow is needed to truly understand the timeline of events.
Therefore, MBI Research Fellow Dr. Mario Mendieta-Serrano and a team of scientists from the lab of MBI Principal Investigator Assoc. Prof. Timothy Saunders used advanced high-resolution microscopy and image processing to follow muscle tissue development in live zebrafish embryos for at least 16 hours. Zebrafish are often used for developmental studies as they are one of the simplest vertebrates (organism with a backbone or spine), and are optically transparent so external development can be monitored using a microscope (Video 1).
The researchers then manually imaged and tracked individual slow and fast muscle cells to create a map of muscle cell rearrangement, combined with cell shape analysis for identifying elongation or cell fusion events. Importantly, this map is 4 dimensional, providing both the 3D spatial location of a cell as well as its location over time (Video 2).
Remarkably, their initial cell-level analysis revealed that the cell fusion events that lead to fast muscle cell differentiation randomly occurred in both space and time. Cell fusion events occurred all over the developing muscle and were not confined to a specific 3D location and took place over a long 5-hour window, not at a specific time point. Further analysis of cell shape or elongation ruled out either of these morphological factors as an indicator of cell fusion. Given that muscle tissue will robustly develop a striated pattern, the high level of variation in cell fusion and lack of any controlling factor puzzled the researchers.
However, observation of muscle development at the tissue-level started to provide some answers. They saw a wave of fusion events along the left to right (mediolateral) axis of the embryo, demonstrating that a pattern is formed. Next, the team searched for factors linked to fusion at the tissue level. They found that expression of a protein that promotes cell fusion named Myomaker correlated with the cell fusion location, although it did not determine the occurrence of the fusion events. By comparing the movement of slow muscle fibres with fast muscle fusion, they were able to uncover a strong correlation where the slow muscle fibres appear as ‘train tracks’ that guide fast muscle cells to regions where they can fuse together. Further evidence for this hypothesis came from experiments where slow muscle fibres were removed, leading increased variability in spatial location and timing of fast muscle fusion. With these two correlating factors, they were able to develop a model where Myomaker expression defines an area where fusion can take place, and slow muscle fibres migration increase the likelihood of fusion occurring. Although neither of these two factors directly control cell fusion, which remains a randomly occurring event, they work together to accurately predict the cell fusion pattern observed in the zebrafish embryo.
This paper describes how random cellular events during muscle development can be regulated by biochemical and biomechanical interactions to drive robust pattern formation at the tissue level. While this process is resilient, when disrupted it can lead to failure of correct tissue structure formation and developmental disease. Fortunately, the quantitative 4D mapping approach described here by the research team can be readily applied to study development of other organs.