Forcing apart the dual role of formin and profilin
Mechanical and biochemical regulation of actin filament polymerization
Edited by Andrew MS Wong, PhD. Illustration by Diego Pitta de Araujo, PhD | 03 Aug 2018
A research team led by Prof. Jie Yan, Principal Investigator at the Mechanobiology Institute (MBI) and Department of Physics, National University of Singapore, has revealed how the growth of actin filaments is regulated; mechanically by force and biochemically by profilin, and how this interplay enables dynamic structural rearrangement of the cell in response to stimuli. This study was published in Nano Letters.
Unravelling actin polymerization through single-molecule manipulation
Every cell is internally supported by the actin cytoskeleton, a meshwork of actin filaments and myosin motor proteins that acts as a structural scaffold, much like the steel frame of a skyscraper. However, as actin filaments are made up by polymerization of actin monomers this enables the cytoskeleton to be highly dynamic – filaments can grow, shrink, branch off, and even bundle together. The organization of the dynamic cytoskeleton is tightly controlled by a variety of proteins, and one such family are formins.
Formins are critical for promoting actin nucleation (initiation of new filaments) and for regulating polymerization (elongation of an existing filament). Formins have a FH1 and FH2 domain, and assemble as homodimers (i.e. two individual formins bind to each other). The dimerized FH2 domains assemble into a ring-like structure, which encircles the barbed end of an actin filament where polymerization occurs. As the filament elongates, it rotates relative to the FH2 ring. The FH1 domains extend from the FH2 ring like a tail, and contain amino acids which have an attraction to profilin, a protein that binds to actin monomers. Therefore, the two FH1 domain of the homodimer are thought to enrich the local concentration of actin monomers in the vicinity of the barbed end, effectively bringing the actin monomers to the building site for actin filament growth.
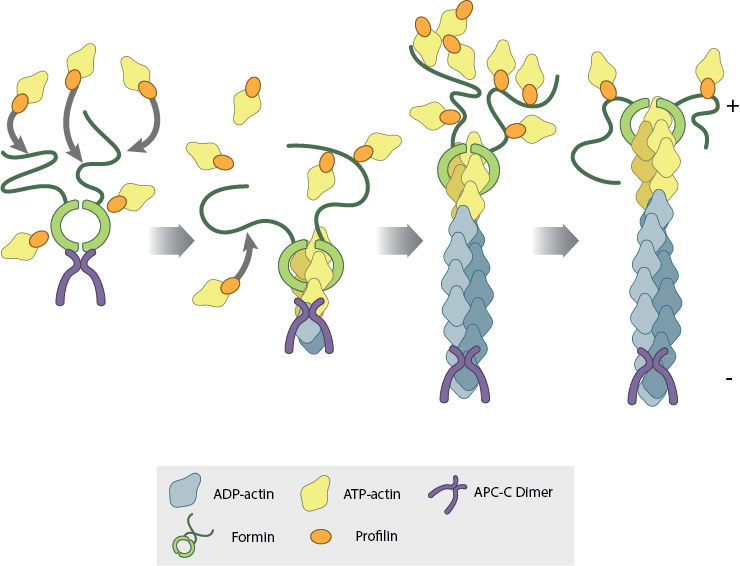
Illustration: Formin regulates actin filament polymerization through both of its domains. The dimerized FH2 domain assembles as a ring (light green) around the barbed end of the filament where polymerization takes place. The FH1 domain (dark green) attracts profilin (orange) bound to actin monomers (yellow), bringing them to the barbed end for filament elongation. Image adapted from www.mechanobio.info. A stylized version of this schematic appears on the cover page.
Within the cell, actin polymerization is subject to mechanical forces and rotational constraints. The contractile activity of myosin motor proteins generates mechanical force that is transmitted to the actin filaments, and the actin meshwork requires cross-linking of filaments that inhibits their free rotation. In order to understand how formin and profilin regulate actin polymerization under these constraints, MBI research fellows Miao Yu, Shimin Le, and colleagues used magnetic tweezers to perform single-molecule manipulation experiments. By tethering a single actin filament between a coverslip surface and a superparamagnetic bead, they were able to measure actin polymerization at a nanometer scale, in the presence of force, rotational constraints, profilin, or a combination of these factors.
Formin-mediated actin polymerization is regulated by mechanical force, and profilin concentration. Together, they facilitate rapid and dynamic reorganization of the actin cytoskeleton that allows the cell to adapt to its changing environment.
They discovered that application of force sped up actin polymerization for rotation unconstrained filaments, but rotation constrained filaments were insensitive to force. Interestingly, adding profilin could speed up actin polymerization up to six times faster, irrespective of rotational constraints. However, this acceleration depended on the ratio of profilin to actin monomers, which turned out to be biphasic—the fastest polymerization was seen at a ratio of 4 profilins for every 1 actin monomer, but increasing or decreasing the profilin:actin monomer ratio reduced polymerization speed. Their magnetic tweezer experiments also revealed that the FH1 domain of formin is a simple unstructured polymer, which can stretch, but is unable to fold and unfold in response to force. They also provided clear evidence that the FH2 domain of formin is the one which is primarily responsible for sensing and responding to mechanical forces.
This study reveals how formin mediated actin polymerization can be regulated independently by force and profilin, and how they can work in conjunction to rapidly accelerate filament polymerization as required. This wide spectrum of outcomes provides a way for the cell to rapidly adjust and rearrange the actin cytoskeleton in response to the dynamic environment. As the actin cytoskeleton is involved in various physiological and pathological functions, such as cell migration, differentiation, embryo development, and cancer metastasis, this advance in understanding how formins can sense mechanical changes and regulate actin organization could reveal how formins maintain cytoskeletal integrity in number of biological processes.