Dissecting Focal Adhesions
Steven Wolf | august 2015
The results from this study are published in Proceedings of the National Academy of Sciences U.S.A [Liu J. et al, Talin determines the nanoscale architecture of focal adhesions. PNAS, 2015. pii: 201512025].
Reverse engineering molecular machines
For years biology was studied independently of physics. Despite this, cells and other biological systems live under the influence of physical forces. Physical properties of the environment can define how cells move, grow and form tissues or organs. Cells can also generate their own forces, and do so with such precision that specific processes like tissue formation repeatedly give rise to organisms with well-defined sizes, shapes and appearance. The influence of physics on biological phenomena has presented scientists with a new paradigm in which to understand life. One of the main questions being asked now is what lies inside cells that allow them to respond to the physical world around them. How do cells know when to move, and where to move to? How can cells avoid being damaged by forces that are too strong or too weak to support them?
Molecular rulers of focal adhesions
Following this line of enquiry, a group of scientists led by MBI Principal Investigator A/Prof Pakorn (Tony) Kanchanawong, have focused on picking apart a molecular machine, called focal adhesions that cells use to attach to their environment and measure its physical properties. This is similar to how we use our hands and feet to touch and feel our surroundings. In particular, the researchers wanted to understand how this machine is constructed within the cell and how this construction enables the machine to function, in terms of the way forces are channeled through this machine.
Focal adhesions are located at the cell membrane and provide a link between the outside and inside of the cell. On the inside, the focal adhesion connects to a network of filaments, known as the cytoskeleton. This network will assemble and disassemble according to whether the cell needs structural support, or needs to move. On the outside, the focal adhesion connects to specific proteins in the immediate environment, called matrix proteins. Interestingly, like a clutch that controls the amount of power transmitted from the engines to the wheels of a car, focal adhesions can actively adjust how much force cells apply to their environment. However, the changes that occur at the molecular level have remained unclear.
With an aim to understand how the size or geometry of particular protein components within focal adhesions could affect their architecture and function, the researchers focused on a protein called talin. Previous work by Dr. Kanchanawong has shown that this protein spanned the height of the focal adhesion and probably provided structural support by forming pillars that reached from the membrane to the filament network above.
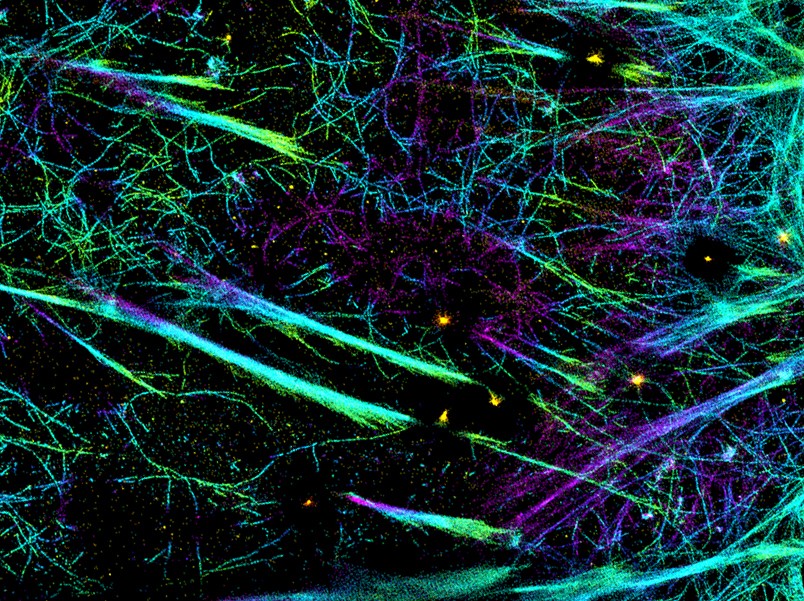
Working on human endothelial cells, the group replaced naturally occurring talin with a set of artificially synthesized talin proteins of varying lengths. This was achieved using protein engineering, where distinct segments of the protein chain were removed, thus producing progressively shorter yet functional proteins. Using super-resolution microscopy to actually see the effect of providing cells with talin molecules that ranged in length, the group revealed just how significant these individual components are in the function of the focal adhesion.
As talins of decreasing lengths were introduced, focal adhesions became shorter, showing that it was talin that was determining the height of the focal adhesion. In terms of geometrical orientation, talins were found to occur diagonally in the focal adhesion, which led the researchers to identify additional roles for the protein. Not only was it acting as a ‘molecular ruler’ to determine the height of a focal adhesion, but it was also absorbing and balancing forces from the cytoskeleton with forces from the environment. In essence, by controlling how much force cells apply to their environment, the specific geometry of talins also enables focal adhesions to function like a ‘molecular clutch’.
These findings are crucial because cells in our bodies are constantly under stress from physical forces. To ensure cells can form and maintain tissue organization, they require molecular machines to absorb and transmit forces, or even generate counter forces. Only now are scientists beginning to understand the significance of the relationship between physics and biology in the development of complex organisms. In this study, super- resolution microscopy has been the essential tool that allowed researchers to see individual components of the focal adhesion and determine their function. With super-resolution microscopy now becoming more widely available, an exciting time lies ahead for science as researchers better understand the engineering principles that cells make use of and how physical phenomena is integrated into biological systems to give rise to the life that we see and feel around us every day.