Development in Time and Space
Shedding light on the importance of time in embryo development
Andrew Wong and Steven Wolf | 18 OCTOBER 2017 | illustration by Anqi Huang
A team of researchers from the Mechanobiology Institute, National University of Singapore have used optogenetics to reveal how protein concentration gradients impart both spatial and temporal information for the determination of cell fate during development. This study is published in eLife. Huang A et al. Decoding temporal interpretation of the morphogen Bicoid in the early Drosophila embryo. Elife. 2017 Jul 10;6. pii: e26258. doi: 10.7554/eLife.26258.

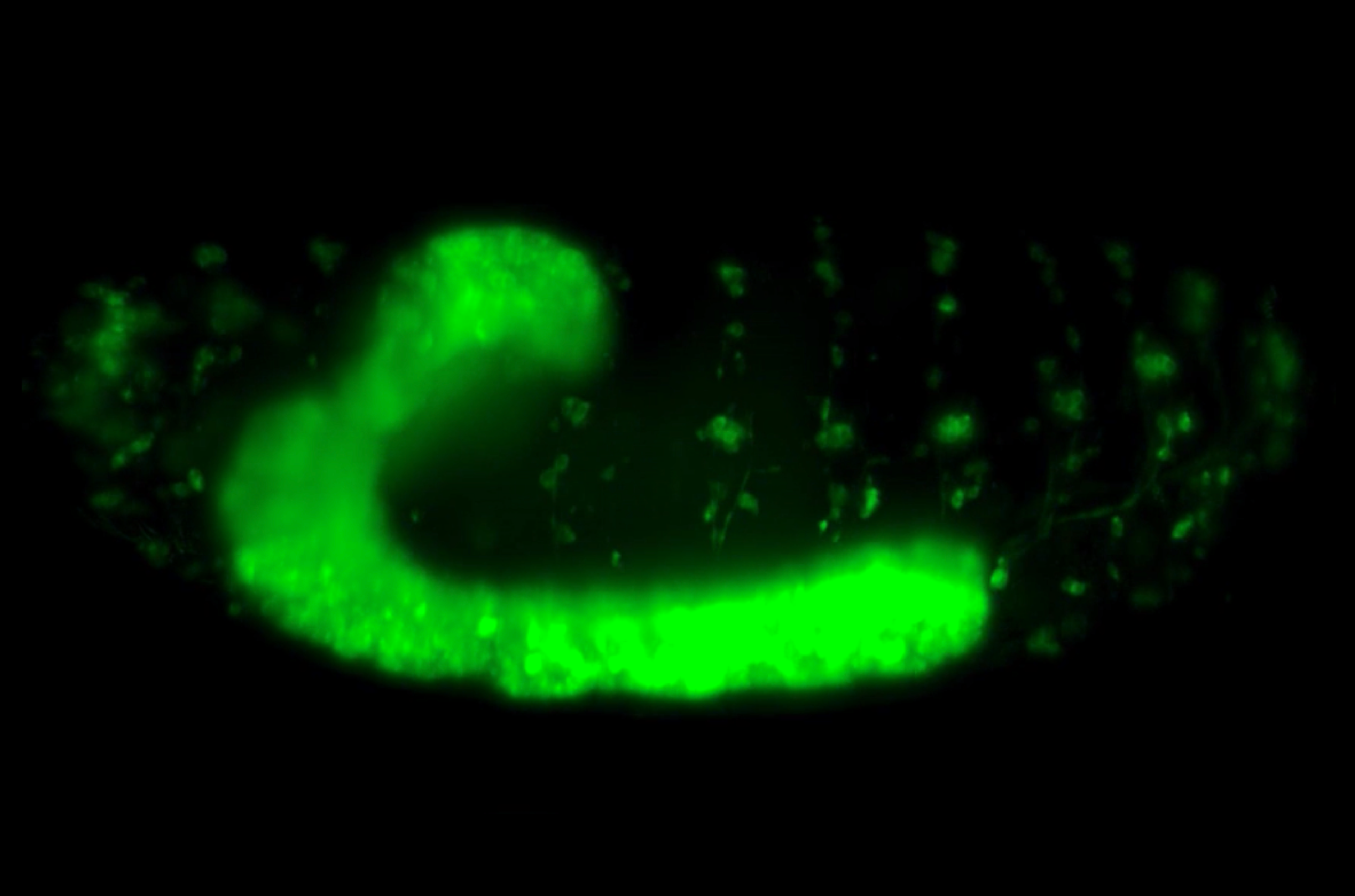
Artistic illustration of a Drosophila embryo undergoing development over time. The numbers refer to the stages of nuclear cleavage divisions. Illustration by Anqi Huang.
Shedding light on the importance of time in embryo development
Biological organisms display a range of patterns and spatial features, from leopard spots to the stripes on fish. Understanding how these patterns emerge at the correct time and place is a major problem in biology. During development cells need to take on specific cell fates. How, where, and when cell fate is determined has been the source of intense study. Alan Turing first posited the idea of a morphogen, a signalling molecule that originates from a single cell before spreading through a tissue, thereby establishing a morphogen gradient, in his seminal 1952 paper “The Chemical Basis of Morphogenesis”. He demonstrated that a wide range of biologically relevant patterns can emerge from surprisingly simple networks of only two morphogens. More recently, it has been demonstrated that embryos can interpret morphogen gradients with remarkable precision, enabling robust embryonic development.
The use of optogenetics to dissect both the spatial and temporal aspects of morphogen gradients is now possible, and promises to unlock the temporal dimension of other similar signalling systems in the future.
The key idea behind the morphogen hypothesis is that certain genes can be “switched on” or “off” depending on the concentration of morphogen input that the cell is exposed to. This input concentration is dependent on where the cell is relative to the morphogen source. However, recent work has revealed that ‘temporal’ or time-based information is also utilised by biological systems – i.e. when the signal is received and interpreted. For example, how long a cell is exposed to a particular morphogen, or whether the signal is detected at a specific time in the sequence of developmental events, can play a role in cell fate determination.
Networks that allow cells to robustly interpret morphogen signals have now been found in a variety of systems, from the early Drosophila (fruit fly) embryo to the development of the vertebrate nerve cord. The first experimentally discovered morphogen is Bicoid, which is a transcription factor that binds to DNA, and forms a gradient in the early Drosophila embryo. The concentration of Bicoid is highest at the anterior pole (front) of the embryo, and decays as it spreads towards the posterior pole (rear).
It has been shown that spatial information from the Bicoid gradient is important in determining the fate of cells in the developing fly embryo. However, determining the influence of temporal information, such as the length of time in which cells are exposed to Bicoid, has been particularly challenging.
In order to address this problem, MBI researchers Anqi Huang and Christopher Amourda from the lab of Asst. Prof Timothy Saunders utilised a method known as optogenetics, to control Bicoid signalling in the developing fruit fly embryo. Optogenetics uses light activation to alter the configuration of light-sensitive proteins. The Saunders lab fused such a light-sensitive protein to Bicoid. When embryos expressing the light-sensitive Bicoid fusion were kept in the dark, Bicoid activity and development proceeded as normal. However, upon activation by blue light, the light-sensitive Bicoid fusion could no longer support transcription, effectively turning off the signalling pathways that depend on Bicoid input. However, the concentration gradient of Bicoid itself was left untouched.
With the ability to precisely control the temporal component of gene activation inside the living embryo, the researchers were able to determine the importance of the timing and duration of Bicoid signalling. Interestingly, switching off Bicoid in the first hour after fertilization did not affect the embryo, but after this stage, interrupting Bicoid activity led to problems or failure in embryonic development. In addition, cells located in the embryo anterior (which are exposed to high concentration of Bicoid), also needed to be exposed to Bicoid for a longer period in order to undergo specialization. Conversely, cells located at the embryo posterior, where Bicoid expression is low, only required a short period of Bicoid exposure before they became specialized.
The importance of time in embryo development has been made clearer from this work. How long cells are exposed to Bicoid, and not just how much Bicoid they are exposed to, impacts cell fate determination. The use of optogenetics to dissect both the spatial and temporal aspects of morphogen gradients is now possible, and promises to unlock the temporal dimension of other similar signalling systems in the future.