Cell arrangements under confined geometries
How epithelial cells arrange in curved environments
Sruthi Jagannathan | FEBRUARY 2018 | Illustration by Diego Pitta de Araujo
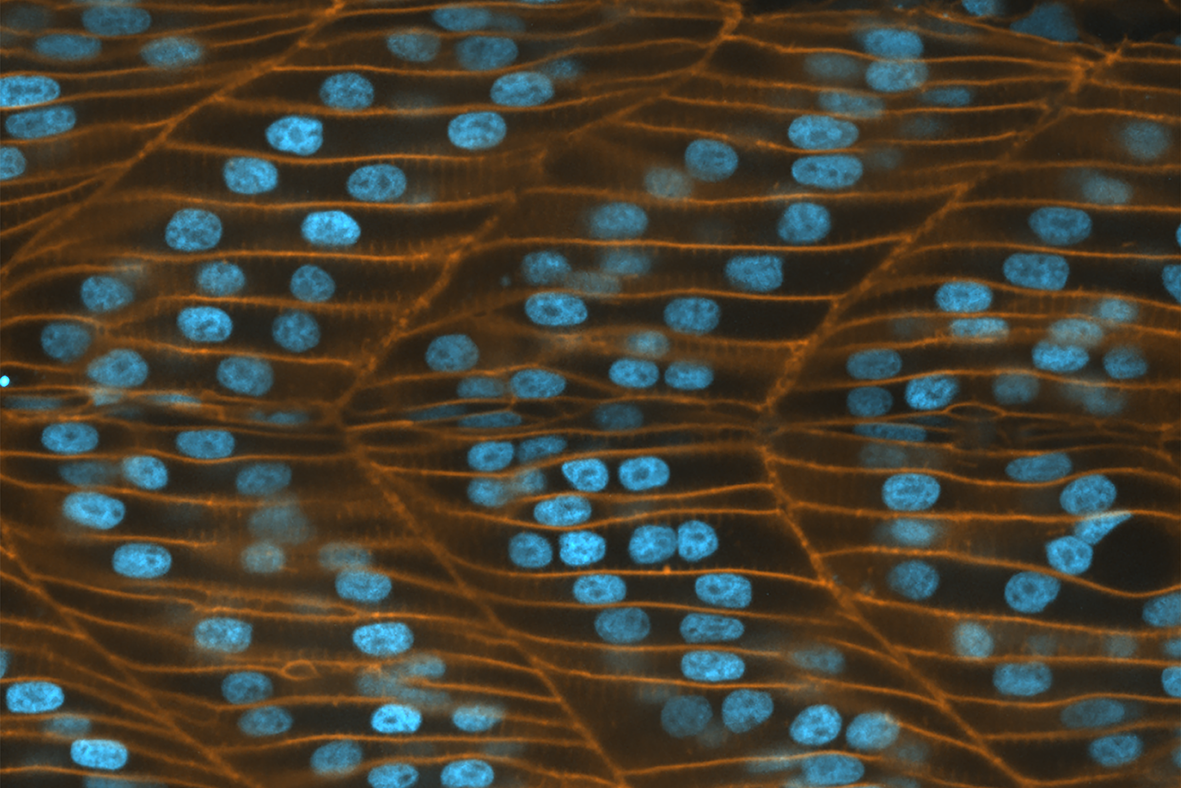
Epithelial tissue organization often occurs under constrained geometries with curved surroundings. Researchers at the Mechanobiology Institute, National University of Singapore describe how epithelial cells undergo changes in their three-dimensional architecture and arrangement patterns, within curved geometries at the Drosophila anterior pole. This study is published in the Molecular Biology of the Cell.
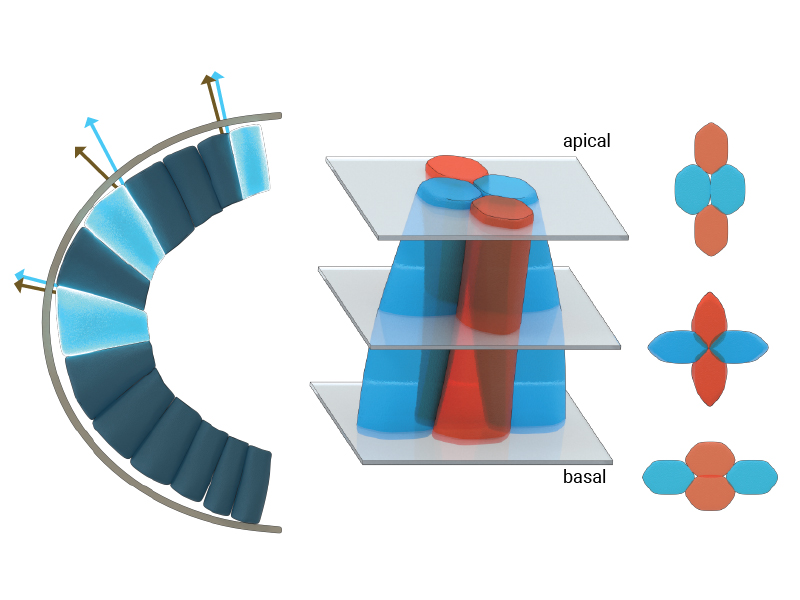
The illustration depicts the three-dimensional morphologies and arrangement of epithelial cells at the anterior pole of the Drosophila embryo. Under the geometric constraints at the curved apical pole, cells skew or deform towards the trunk region, seen by the skew angle between the normal to the apical surface (grey arrows) and the cellular edge (blue arrows). The cells at the anterior pole also frequently exchange their neighbors along their apical-basal axes (seen as blue-blue cell neighbors on the apical side and blue-red cell neighbors on the basal side.
The organization of a curved epithelium
Epithelial tissues are among the first to be formed during embryo development. Organized as sheets of highly interconnected cells, it provides a structural support to the growing embryo and, in the adult organism, lines the cavities and surfaces of organs such as the skin, gut, and respiratory and urinary tracts.
The growing cell sheets are held together tightly by intercellular junctions, which provide mechanical support to the epithelial tissue and enable it to support the developing embryo. However, epithelial tissue can also display fluid-like behavior. This can occur as the epithelial cells divide, change shape, and break and reform intercellular junctions in response to chemical and mechanical signals. Such behavior forms the basis for tissue regeneration in adult organisms and for active remodeling of tissue architecture during morphogenetic events in the developing embryo.
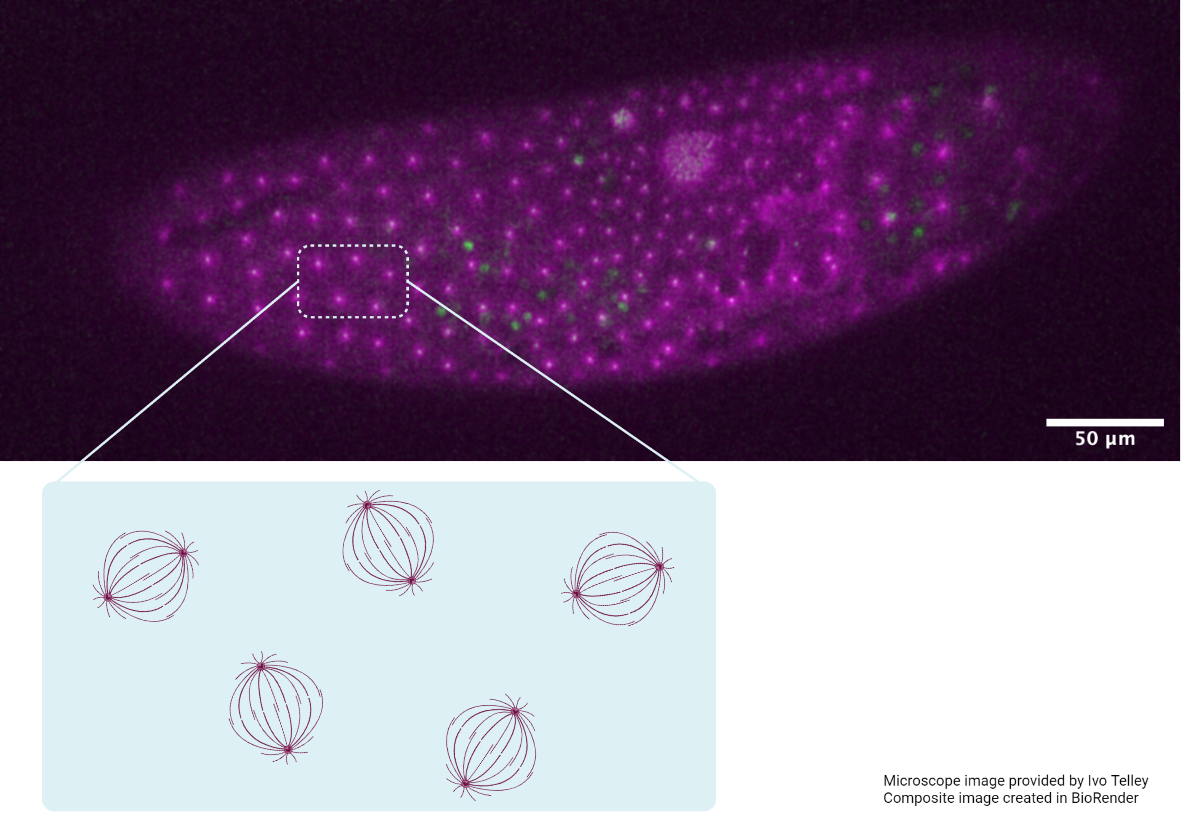
Using confocal microscopy and computational models, we were able to describe how cells pack within under geometric constraints within curved epithelial tissues.
Interestingly, epithelial tissues are able to maintain their structural integrity and function as robust mechanical barriers even as their cells constantly reorganize and acquire complex shapes. It is now clear that the arrangement and dynamics of epithelial cells is critical in regulating the functional homeostasis of the tissue. A large number of experimental and theoretical studies have shown how maximum packing efficiency is achieved when epithelial cells arrange into hexagonal structures.
However, this cell packing model is optimal for tissue organization on a flat environment, which is often not the case for epithelial tissues. Instead, epithelial tissue organization often occurs under constrained geometries with curved surroundings. This is the case in the gut, where cells must be renewed throughout adult life, and at the poles in Drosophila embryos. It is currently unclear how cells compete for space and whether there are changes required in their three-dimensional morphologies and arrangement patterns, within such constrained geometries.
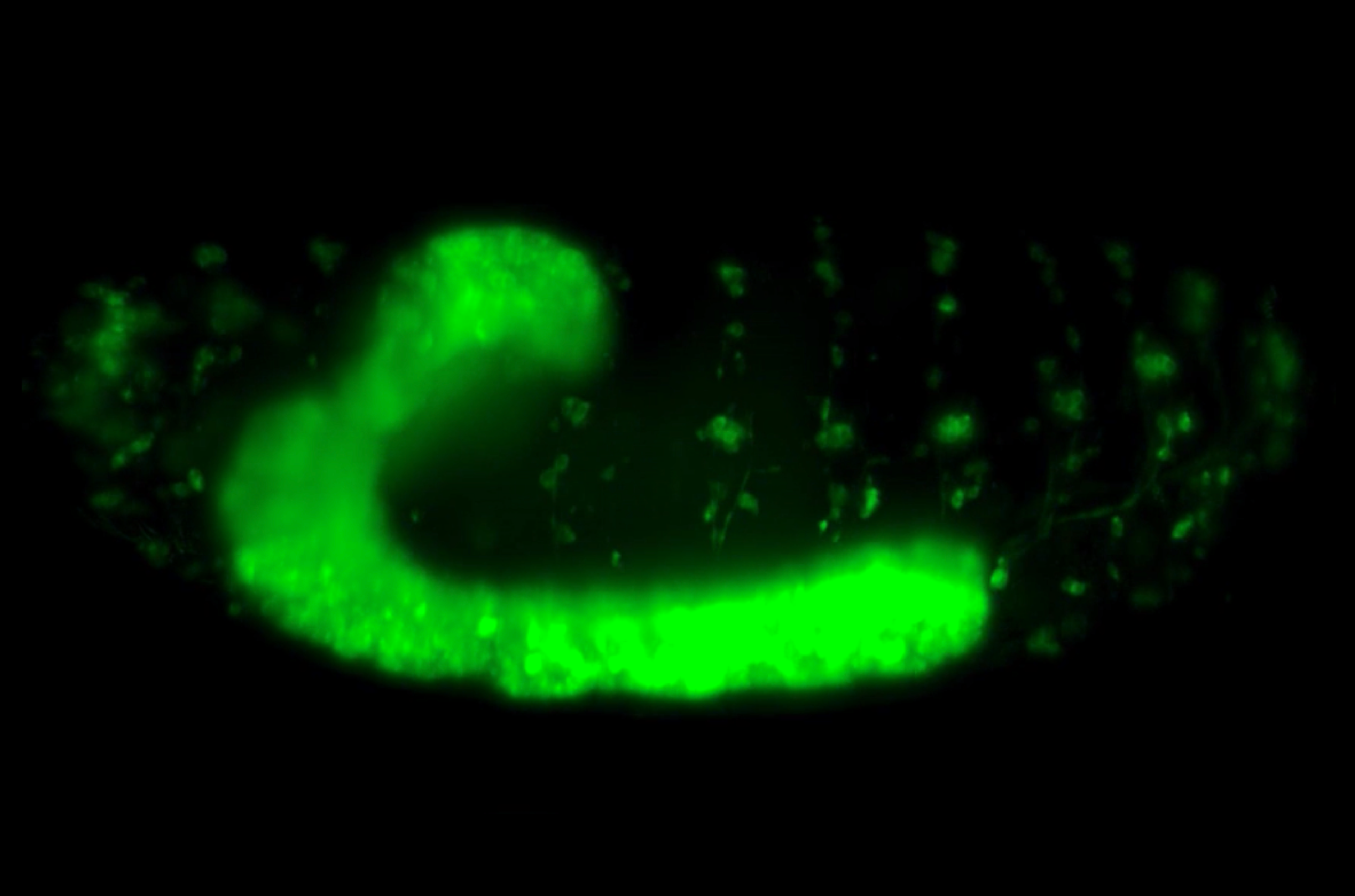
To address these problems, Assistant Professor Timothy Saunders, in collaboration with scientists at the Institute of Molecular and Cell Biology, A*STAR, Singapore, focused on an early event in Drosophila embryogenesis called cellularization. During this event, a sheet of epithelial cells are formed from the multinucleated syncytial embryo due to the simultaneous ingression of cell membrane around each individual nucleus. The newly formed cell sheet is arranged along the surface of a rugby ball-shaped embryo. With their apical (or top) surfaces restricted in growth by a rigid outer embryonic layer, the cells increase their volume by extending their basal (or bottom) surfaces towards the interior of the embryo. In the highly curved region of the embryo head (which corresponds to the tip of the rugby ball), there is limited area for basal surface extension and cells compete for space. However, in the flatter regions of the embryo trunk (which correspond to the center of the rugby ball), cells are able to extend their basal surfaces more freely.
Using confocal microscopy techniques, Saunders and colleagues quantified the evolution of shape and arrangement of cells in both the highly curved region of the head and within the flatter region of the trunk. They found that cells near the embryo head skewed or deformed towards the trunk region, which is geometrically less constrained. A temporal analysis further revealed that cells in the head region changed their neighbors more frequently than cells in the trunk, in a process called a T1 transition. This resulted in cells having different neighbors at their apical and basal surfaces. Interestingly, these T1 transitions mainly occurred in the region of maximum skew along the apical-basal axes of these cells.
Based on analytical calculations, MBI Research Fellow Dr. Jean- Francois Rupprecht predicted that the location of maximum cellular skew is close to the embryo head. In the presence of a cell skew, the growth of the cell-cell membrane contributes to the mechanical balance of the apical tissue. This leads to the hypothesis that the contribution of the growth forces to the apical tissue dynamics is maximal near the embryo head.
To test this hypothesis, Jean-Francois developed a computational model of the tissue called a three-dimensional vertex model. Using this, he found that the growth-induced forces within the constrained geometry are indeed sufficient to drive the observed apical-basal T1 transition near the head.
Previous studies on the mechanics of tissue organization and organ growth have focused on the dynamics of the (two-dimensional) apical surface of epithelial cells. However, findings from this study clearly demonstrate the significance of considering the entire three-dimensional architecture of cells when considering tissues in curved environments. Force balance between the apical and basal surfaces drives the overall tissue dynamics and the onset of cellular processes such as division, growth, and rearrangements during tissue and organ formation.
By shedding light on how curvature affects epithelial tissue organization, this work provides potential insights into the organization of similar biological systems. Examples include the midgut wherein epithelial tissues are found within curved environments, and curvature is known to act as a mechanical signal for the localization of topological defects in these tissues.