Rishita CHANGEDE
Alumni, Senior Research Fellow, Mechanobiology Institute, National University of Singapore
Press
“‘Going dotty’ in service of science,” The Straits Times, Jan 15,1016
Other Activities
Organizing member Women in Science @ MBI
Teaching, selected ‘Special Topics in Mechanobiology’
When spacing matters
Dr Rishita Changede of the Sheetz Lab is exploring how the spacing between matrix protein fibers affect clustering of the cell surface receptors integrins and how this influences the formation of integrin-mediated cell-matrix adhesions and subsequent cell spreading. Learn more
Women in Science Special Segment Explores Gender Equality in STEM
As part of the recently held 2017 Joint Symposium on Bioimaging between Singapore and Japan, a Women in Science segment was organized to explore gender equality issues in STEM.
Adhesion ABC
Integrin clusters are the universal units of cell adhesion
Rishita Changede
Alumni
Principal Investigator
Research Interests
Molecular Mechanisms of Mechanobiology
Dr. Changede is interested in understanding the fundamental designs that are used in biological systems to bring about a well thought out response to the varied physical and biochemical signals that the cells see. This defines the fundamental nature of systems to be able to adapt to ever changing environments for survival using the available machinery.
Fibroblasts are the major cells of the connective tissue. Connective tissue surround all tissues and hence have rigidities varying from few kPa(brain) to MPa(bone). What are the design principles that help the fibroblast to respond to these vastly varying substrates in the body?
Research Areas
Several important biological processes such as Cell-matrix adhesions, Cell migration, Cell signaling are at the interphase of chemical and physical signals that the cell receives and responds to. What are the underlying fundamental principles that govern signal integration of biochemical and physical signals to bring about these important biological processes?
Biography
Dr. Changede is a senior research fellow at MBI working with Prof. Michael Sheetz. Her primary interest is to understand the design principles that allow the cells and organisms to respond appropriately to their diverse physical (geometry and forces) and chemical (biochemical ligands) environment. During her postdoctoral studies, she developed quantitative super resolution imaging based assays to understand the emergent properties of nascent adhesions. Her Ph.D studies in the laboratory of Prof. Pernille Rorth, were focused on developing biosensors to observe the endogenous signaling of guidance receptors (which guide cell migration) in a live tissue. Subcellular localization of this weak endogenous signal was observed at the leading edge of the cell and it was found to be dynamic in both space and time. Her masters thesis was with Prof. Krishanu Ray at Tata Institute of Fundamental Research, India. She studied the role of dynein dynactin complex in endomembrane assembly.
Education
2013 PhD from the National University of Singapore, completing her doctoral thesis at Institute of Molecular and Cell Biology at ASTAR, Singapore
Current Projects
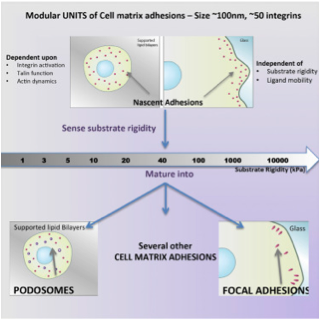
Cell matrix adhesions are made of modular units of densely packed integrins
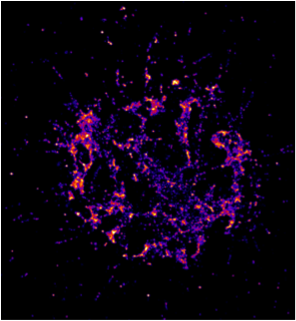
Nascent adhesions observed by Photoactivated Light microscopy (PALM) in mouse embryonic fibroblasts spread on supported lipid bilayers conjugated to RGD.
When fibroblasts spread on fibronectin coated glass they form large focal adhesions whereas they form podosomes like adhesions when they are presented with fibronectin ligands (cyclic Arginine- Glycine- Aspartate -RGD) on supported lipid bilayers. Bilayers are fluid and hence do not support the development of force in the system. How do cells respond appropriately to these substrates?
Using this difference, Dr. Changede asked what is the role of traction force in the extracellular matrix versus intracellular components in formation of these different adhesions? Early events i.e. mechanosensing and formation of early adhesions mediated in minutes time scale were of particular interest as these would mediate the later events of mechanotransduction and mechanoresponse. Using advanced techniques in super resolution microscopy, the formation of diffraction limited nascent adhesions was analyzed. Techniques to quantify the number of molecules within these dense early adhesions were developed. Surprisingly, it was found that very similar nascent adhesions – 100nm discs containing about 50 integrins are laid out on matrices of varying rigidities. The size of these adhesions was very robust at 100-120nm. These evidences suggest that these are modular units of adhesion, which are the first biochemical response of the cell upon ligand binding. These modules are then used by the cell to sense the physical forces in the extracellular matrices. Large adhesions were also observed to be loose aggregates of these dense nanometer scale integrin clusters.
How do clusters of this robust size assemble?
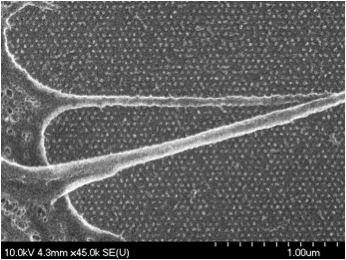
Scanning electron micrograph of mouse embryonic fibroblasts spreading on gold nano patterned hexagonal arrays functionalized with RGD (Patterning with Dr. Haogang Cai).
Open question of how the size of these adhesions is regulated is being studied using gold nano patterned substrates. These substrates allow to present ligands in a restricted fashion at the nanometer length scale. Formation of nascent adhesions on glass, where the ligand is immobile is suggests that unliganded integrins are being recruited to these adhesions. Gold nano substrates (using electron beam lithography), which can present very few ligands is being used to test this. These substrates are further used to address the important question of function of these modular units.
How do different ligands can produce differences in force sensing?
Earlier studies have shown that fibroblasts cannot spread on fibronectin coated soft substrates (generated by PDMS). However, the cell can spread if it is presented with both fibronectin and collagen showing that cell spreading is not dependent solely on extracellular traction forces. This raises the intriguing question of how the cells mount these different responses? Using micro fabrication (UV lithography) we are presenting the cell with two different substrates to understand what helps the cell to determine its response. This would be important, as fibroblasts in the connective tissues in the brain would respond very differently from fibroblasts in the skin or bone.
How is cell matrix adhesion formation modulated from inside the cell?
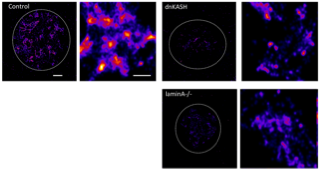
MEFs expressing the mutants were spread on supported lipid bilayers functionalized with RGD. Integrin mediated RGD (Dylite 650) clusters underneath the cells were imaged using PALM. Each panel shows a single cell (dotted line). Adjacent panel shows a zoom in region of a small region of the cell. Scale: 2um and 200nm respectively.
Diseases such as cancer or progeria start with a mutations inside the cell. How does this regulate the formation of cell matrix adhesions? The hypothesis here is that the predisposed mechanical state of the cell will regulate the type of adhesions that the cell can form. How is formation of cell matrix adhesion regulated by changes within the cell.
In collaboration with Prof. G.V. Shivashankar’s lab using mutants expressing progerin or mutants of the molecular links between the nuleoskeleton and cytoskeleton (lamin A/C and KASH domain proteins), we are addressing how it regulates cell-matrix adhesions.
Recent Publications
- Jain K, Kishan K, Minhaj RF, Kanchanawong P, Sheetz MP, and Changede R. Immobile Integrin Signaling Transit and Relay Nodes Organize Mechanosignaling through Force-Dependent Phosphorylation in Focal Adhesions. ACS Nano 2025;. [PMID: 39760672]
- Lin S, Changede R, Farrugia AJ, Bershadsky AD, Sheetz MP, Prost J, and Rupprecht J. Membrane Tilt Drives Phase Separation of Adhesion Receptors. Phys Rev Lett 2024; 132(18):188402. [PMID: 38759206]
- Jain K, Minhaj RF, Kanchanawong P, Sheetz MP, and Changede R. Nano-clusters of ligand-activated integrins organize immobile, signalling active, nano-clusters of phosphorylated FAK required for mechanosignaling in focal adhesions. bioRxiv 2024;. [PMID: 38464288]
- Jain K, Pandey A, Wang H, Chung T, Nemati A, Kanchanawong P, Sheetz MP, Cai H, and Changede R. TiO2 Nano-Biopatterning Reveals Optimal Ligand Presentation for Cell-Matrix Adhesion Formation. Adv Mater 2024;:e2309284. [PMID: 38340044]
- Jain K, Lim KYE, Sheetz MP, Kanchanawong P, and Changede R. Intrinsic self-organization of integrin nanoclusters within focal adhesions is required for cellular mechanotransduction. bioRxiv 2023;. [PMID: 38045378]
- Jain K, Kanchanawong P, Sheetz MP, Zhou X, Cai H, and Changede R. Ligand functionalization of titanium nanopattern enables the analysis of cell-ligand interactions by super-resolution microscopy. Nat Protoc 2022;. [PMID: 35896742]
- Bonnard C, Navaratnam N, Ghosh K, Chan PW, Tan TT, Pomp O, Ng AYJ, Tohari S, Changede R, Carling D, Venkatesh B, Altunoglu U, Kayserili H, and Reversade B. A loss-of-function NUAK2 mutation in humans causes anencephaly due to impaired Hippo-YAP signaling. J. Exp. Med. 2020; 217(12). [PMID: 32845958]
- Changede R, Cai H, Wind SJ, and Sheetz MP. Integrin nanoclusters can bridge thin matrix fibres to form cell-matrix adhesions. Nat Mater 2019;. [PMID: 31477904]
- Saxena M, Changede R, Hone JC, Wolfenson H, and Sheetz MP. Force induced calpain cleavage of talin is critical for growth, adhesion development and rigidity sensing. Nano Lett. 2017;. [PMID: 29052994]
- Saxena M, Liu S, Yang B, Hajal C, Changede R, Hu J, Wolfenson H, Hone J, and Sheetz MP. EGFR and HER2 activate rigidity sensing only on rigid matrices. Nat Mater 2017;. [PMID: 28459445]
Selected Publications
Nanoclustering of integrins on fluid substrates depends on Talin function. Rishita Changede, Xiaochun Xu, Felix Margadant, Michael P. Sheetz; Developmental Cell. 2015 Dec 7; 35(5) 614.
EGFR Activates Rigidity Sensing Only on Rigid Matrices with or Without Ligand Shuimin Liu*, Mayur Saxena*, Rishita Changede, Cynthia Hajal, Bo Yang, James Hones, Haguy Wolfenson, Michael P. Sheetz. (under revision at Nature Materials)
Force-dependent talin cleavage by calpain is critical for rigidity sensing and nascent adhesion regulation. Mayur Saxena, Rishita Changede, Haguy Wolfenson, Michael P. Sheetz. under revision at Nature Communications.
Integrin and Cadherin Clusters: A Robust Way to OrganizeAdhesions for Cell Mechanics. Rishita Changede, Michael P. Sheetz. Bioessays – under revision
Sensitive detection of active RAS for quantitative analysis of guided migration of Drosophila border cells. Changede et al.; under submission.
Cell behaviors regulated by guidance cues in collective migration of border cells. Poukkula M, Cliffe A, Changede R, Rørth P. J Cell Biol. 2011 Feb 7;192(3):513
Expression pattern of Drosophila translin and behavioral analyses of the mutant Suseendranathan K, Sengupta K, Rikhy R, D’Souza JS, Kokkanti M, Kulkarni MG, Kamdar R, Changede R, Sinha R, Subramanian L, Singh K, Rodrigues V, Rao BJ, Eur J Cell Biol. 2007 Mar;86(3):173