Kabir H BISWAS
Alumni, Mechanobiology Institute, National University of Singapore
mbikhb@nus.edu.sg
kabir.biswas@live.com
Level 09 T-Lab
National University of Singapore
5A Engineering Drive 1
Singapore 117411
Laboratory website
Kabir H Biswas Research
Persistent a-Catenin Activation
Switching behavior of a-Catenin at E-cadherin adhesions
Active Junction Formation
E-chadherin mobility regulates adhesion formation
Kabir H Biswas
MBI Alumni, June 2017
Principal Investigator
Research Interests
Molecular Mechanics of Mechanotransduction Group, Technology Innovation for Mechanobiology Group
Dr Biswas is interested in understanding mechanisms of cellular signal transduction mediated by cell membrane localized as well as cytosolic proteins. For instance, cells in a tissue receive both chemical (e.g. hormones or growth factors) as well as physical (e.g. mechanical tension or cell shape) cues that they need to appropriately process for both survival and growth of the whole organism. He believes that a comprehensive understanding of these processes in the cellular context will be useful in designing therapeutic strategies for alleviation of disease conditions.
Research Areas
Cell signaling, protein function regulation, cell adhesion, biomolecular assay development, biochemistry, biophysics, cell biology, bioinformatics
Biography
Dr Biswas joined the laboratory of Prof. Jay T Groves at the Mechanobiology Institute (MBI), National University of Singapore (NUS) as Research Fellow in December 2011. He has developed assays for E-cadherin adhesion, signaling and crosstalk with other receptors using synthetic, micropatterned membranes and has uncovered a novel, nucleation-dependent mechanism of adhesion formation. Prior to coming to Singapore, he was an Integrated PhD (MS + PhD) student at the Indian Institute of Science (IISc), Bangalore and worked with Prof Sandhya S. Visweswariah, Department of Molecular Reproduction, Development and Genetics (MRDG) towards his Masters as well as PhD thesis. There, he led a project that dealt on the possibility of multiple modes of allosteric regulation of proteins that are involved in cyclic-guanosine monophosphate (cGMP) signal transduction pathway such as guanylyl cyclases and cGMP phosphodiesterases.
Education
PhD Indian Institute of Science
Current Projects
E-cadherin junction formation involves an active kinetic nucleation process
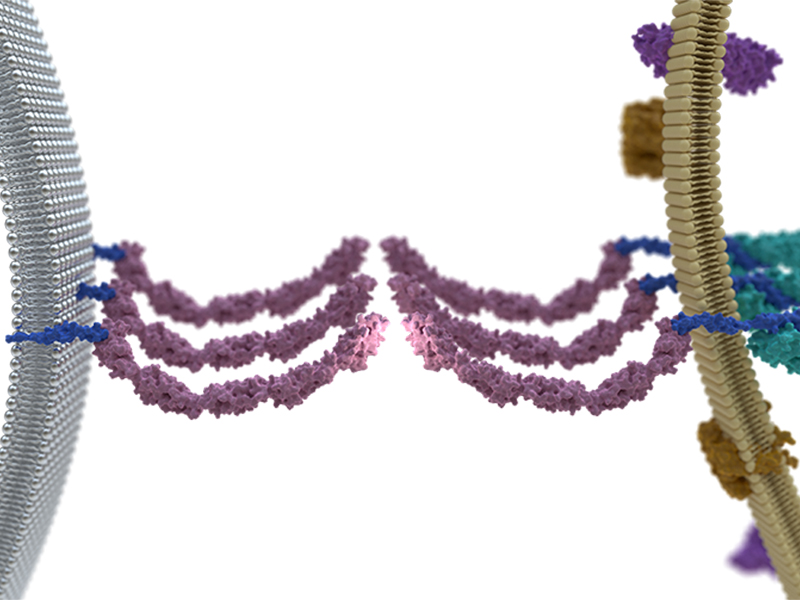
 Biswas KH, Hartman KL, Yu C-h, Harrison OJ, Song H, Smith AW, Huang WYC, Lin W-C, Guo Z, Padmanabhan A, Troyanovsky SM, Dustin ML, Shapiro L, Honig B, Zaidel-Bar R, Groves JT (2015) Proc Natl Acad Sci U S A 112(35):10932-7
Biswas KH, Hartman KL, Yu C-h, Harrison OJ, Song H, Smith AW, Huang WYC, Lin W-C, Guo Z, Padmanabhan A, Troyanovsky SM, Dustin ML, Shapiro L, Honig B, Zaidel-Bar R, Groves JT (2015) Proc Natl Acad Sci U S A 112(35):10932-7
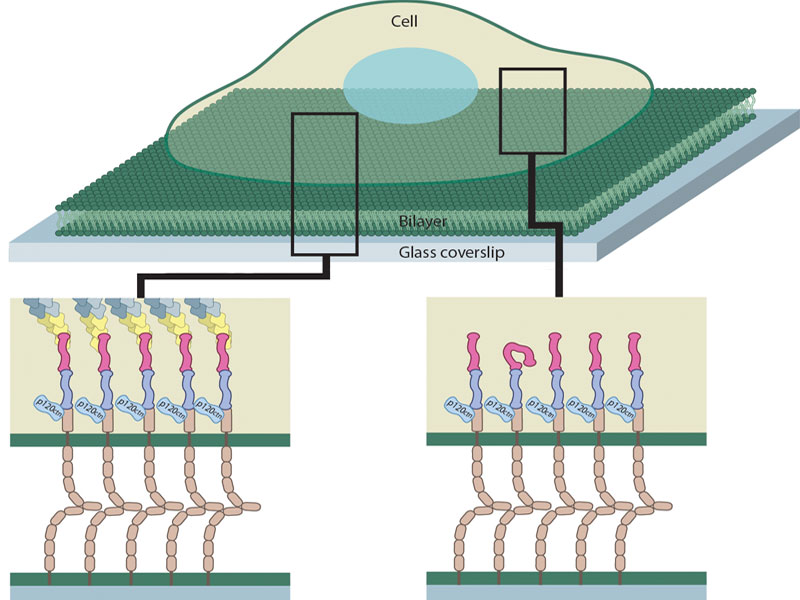
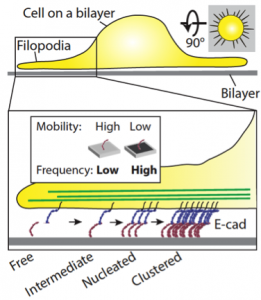
A schematic representation of E-cadherin junction formation on a supported lipid bilayer. Cells seeded on a bilayer interact with E-cadherin by extending and retracting filopodia. Retracting filopodia increases the local concentration of interacting E-cadherin molecules on high viscosity, low mobility bilayers. This process most often fails on low viscosity, high mobility bilayers due to faster diffusive dissipation of E-cadherin molecules.
Distinct Allostery Induced in the Cyclic GMP-binding, Cyclin GMP-specific Phosphodiesterase (PDE5) by Cyclic GMP, Sildenafil and Metal Ions
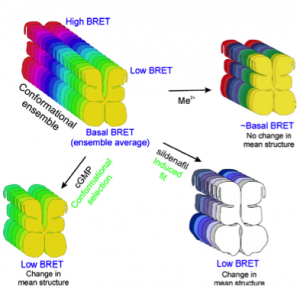 Biswas KH and Visweswariah SS (2011) J Biol Chem 286(10):8545-54
Biswas KH and Visweswariah SS (2011) J Biol Chem 286(10):8545-54
Conformational transitions in PDE5. An ensemble of conformations of PDE5 can exist, with a spectrum of BRET, and the basal BRET being the average. Cyclic GMP selectively binds the low BRET conformers shifting the equilibrium toward reduced average BRET. Sildenafil induces conformational changes in the protein which require high activation energy resulting in a reduction in BRET. A change in the mean structure of the protein occurs in both the cases. Metal ions binding to the catalytic site alter the dynamics of the protein without causing any considerable change in the mean structure. Sequential binding of sildenafil and cGMP, and vice versa, further changes the mean structure of the protein, which for simplicity, is not depicted in the figure. Metal ion binding to the catalytic site reverts the cGMP-bound low BRET conformers to ~basal BRET conformers. In contrast, metal ion binding to the catalytic site enhances sildenafil-induced reduction in BRET.
Recent Publications
- Oh D, Chen Z, Biswas KH, Bai F, Ong HT, Sheetz MP, and Groves JT. Competition for shared downstream signaling molecules establishes indirect negative feedback between EGFR and EphA2. Biophys J 2022;. [PMID: 35430415]
- Chen Z, Oh D, Biswas KH, Zaidel-Bar R, and Groves JT. Probing the effect of clustering on EphA2 receptor signaling efficiency by subcellular control of ligand-receptor mobility. Elife 2021; 10. [PMID: 34414885]
- Huang WYC, Alvarez S, Kondo Y, Lee YK, Chung JK, Lam HYM, Biswas KH, Kuriyan J, and Groves JT. A molecular assembly phase transition and kinetic proofreading modulate Ras activation by SOS. Science 2019; 363(6431):1098-1103. [PMID: 30846600]
- Biswas KH, Cho N, and Groves JT. Fabrication of Multicomponent, Spatially Segregated DNA and Protein-Functionalized Supported Membrane Microarray. Langmuir 2018;. [PMID: 30032610]
- Chen Z, Oh D, Biswas KH, Yu C, Zaidel-Bar R, and Groves JT. Spatially modulated ephrinA1:EphA2 signaling increases local contractility and global focal adhesion dynamics to promote cell motility. Proc. Natl. Acad. Sci. U.S.A. 2018;. [PMID: 29866846]
- Vafaei S, Tabaei SR, Biswas KH, Groves JT, and Cho N. Dynamic Cellular Interactions with Extracellular Matrix Triggered by Biomechanical Tuning of Low-Rigidity, Supported Lipid Membranes. Adv Healthc Mater 2017;. [PMID: 28371558]
- Biswas KH, and Zaidel-Bar R. Early events in the assembly of E-cadherin adhesions. Exp. Cell Res. 2017;. [PMID: 28237244]
- Biswas KH, Hartman KL, Zaidel-Bar R, and Groves JT. Sustained α-catenin Activation at E-cadherin Junctions in the Absence of Mechanical Force. Biophys. J. 2016; 111(5):1044-52. [PMID: 27602732]
- Biswas KH, and Groves JT. A Microbead Supported Membrane-Based Fluorescence Imaging Assay Reveals Intermembrane Receptor-Ligand Complex Dimension with Nanometer Precision. Langmuir 2016; 32(26):6775-80. [PMID: 27264296]
- Yu C, Rafiq NBM, Cao F, Zhou Y, Krishnasamy A, Biswas KH, Ravasio A, Chen Z, Wang Y, Kawauchi K, Jones GE, and Sheetz MP. Integrin-beta3 clusters recruit clathrin-mediated endocytic machinery in the absence of traction force. Nat Commun 2015; 6:8672. [PMID: 26507506]
Publications
- Vafaei S, Tabaei SR, Biswas KH, Groves JT, and Cho N. Dynamic Cellular Interactions with Extracellular Matrix Triggered by Biomechanical Tuning of Low-Rigidity, Supported Lipid Membranes. Adv Healthc Mater 2017;. [Accepted] [PMID: 28371558]
- Biswas KH* and Viswesweriah SS* (2017) Buffer NaCl concentration regulates Renilla luciferase activity and ligand-induced conformational changes in the BRET-based PDE5 sensor Matters [Accepted] [PMID: awaited]
- Biswas KH* (2017) Allosteric regulation of proteins: a historical perspective on the development of concepts and techniquesResonance 22(1): 37-50 [PMID: not available]
- Biswas KH*, and Zaidel-Bar R*. Early events in the assembly of E-cadherin adhesions. Exp. Cell Res. 2017;. [PMID: 28237244]
- Biswas KH*, Hartman KL, Zaidel-Bar R*, and Groves JT*. Sustained α-catenin Activation at E-cadherin Junctions in the Absence of Mechanical Force. Biophys. J. 2016; 111(5):1044-52. [PMID: 27602732]
- Biswas KH*, and Groves JT*. A Microbead Supported Membrane-Based Fluorescence Imaging Assay Reveals Intermembrane Receptor-Ligand Complex Dimension with Nanometer Precision. Langmuir 2016; 32(26):6775-80. [PMID: 27264296]
- Yu C, Rafiq NBM, Cao F, Zhou Y, Krishnasamy A, Biswas KH, Ravasio A, Chen Z, Wang Y, Kawauchi K, Jones GE, and Sheetz MP. Integrin-beta3 clusters recruit clathrin-mediated endocytic machinery in the absence of traction force. Nat Commun 2015; 6:8672. [PMID: 26507506]
- Biswas KH, Hartman KL, Yu C, Harrison OJ, Song H, Smith AW, Huang WYC, Lin W, Guo Z, Padmanabhan A, Troyanovsky SM, Dustin ML, Shapiro L, Honig B, Zaidel-Bar R, and Groves JT. E-cadherin junction formation involves an active kinetic nucleation process. Proc. Natl. Acad. Sci. U.S.A. 2015; 112(35):10932-7. [PMID: 26290581]
- Biswas KH*, Badireddy S, Rajendran A, Anand GS and Viswesweriah SS* (2015) Cyclic nucleotide binding and structural changes in the isolated GAF domain of Anabaena adenylyl cyclase, CyaB2PeerJ 3:e882; DOI 10.7717/peerj.882 [PMID: 25922789]
* co-corresponding author