What is caveolar endocytosis?
Caveolar endocytosis is a clathrin-independent endocytic process which involves bulb-shaped, 50-60nm plasma membrane invaginations called caveolae (or ‘little caves’). Caveolae formation is driven by integral membrane proteins called caveolins as well as peripheral membrane proteins called cavins (reviewed in [1]).
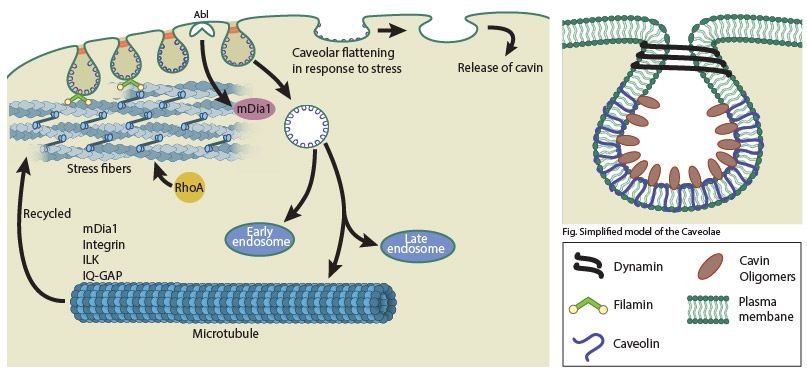
A schematic depicting caveolar endocytosis.
Components of the Caveolae
Caveolins and cavins form the caveolar coat complex which may be responsible for the spiked coat or striations seen around caveolae under an electron microscope [2]. Three types of caveolins are known; caveolin-1 (CAV1) and caveolin-2 (CAV2) found in non-muscle cells, and the muscle-specific CAV3. Each caveolae has around 140-150 CAV1 molecules. Caveolins possess a hairpin domain embedded within the membrane while both the amino and carboxy terminus face the cytoplasm. Although caveolins were considered to be the only proteins responsible for caveolae formation until recently, work in the last decade has identified the cavin family of proteins as integral structural and functional components of caveolae.
Four types of mammalian cavin proteins are known – cavin1 (PTRF), cavin2 (SDPR), cavin3 (PRKCDBP) and the muscle-specific cavin4 (MURC). About 50 cavin molecules are known to associate with each caveolae [3]. All cavins have sequence homology and possess conserved α-helical secondary structures called helical region 1 (HR1) and HR2. Cavins form homo or hetero oligomers with each other with cavin1 being the major and essential component of the oligomers [reviewed in [1]. Other proteins like the GTPase dynamin, dynamin-like ATPase EHD2 and the BAR-domain containing protein PACSIN2 as well as lipids like cholesterol, phosphotidyl serine and PIP2 are also found to be associated with caveolae.
Caveolar endocytosis
Although caveolae were previously shown to be highly immobile and non- endocytic under normal conditions [4], recent studies have established that caveolae are dynamic endocytic carriers ([5],reviewed in [6]). In caveolar endocytosis, caveolae bud off from the plasma membrane and while some attempt to fuse back with the plasma membrane, the majority reach the early endosome before being recycled back to the plasma membrane. Although a novel caveolar endocytic pathway was described involving the caveosome [7], a more recent study revealed that caveosomes are in fact late endosomes modified by overexpression of caveolin1 [8]. The budding of caveolae is mediated by dynamin whereas EHD2 negatively regulates caveolar endocytosis [9],[10]. Albumin, folic acid, viruses like SV40 and EV1, cholera toxin B, and glycosphingolipid analogs are some of the cargo internalized and endocytosed via caveolae.
Caveolae, cytoskeleton and mechanosensing
The cell cytoskeleton plays a role in caveolar organization and trafficking. Actin stress fibers influence the linear distribution of caveolae at the plasma membrane in many cell types. Stress fibers regulated by the tyrosine kinase Abl and the formin mDIA1 play a major role in caveolar organization as well as endocytic trafficking initiated in response to loss of cell adhesion from the substrate [11]. The actin-binding protein Filamin A also plays a crucial role in trafficking of caveolae linked to actin [12]. Microtubules promote recycling of caveolae through local stabilization of microtubules by β1 integrins and integrin-linked kinase (ILK) signaling (reviewed in [13]). The β1 integrin-ILK recruits the actin-binding protein IQGAP1 which together with mDIA1 stabilize microtubules. Thus mDIA1 which regulates both actin and microtubules is crucial for both the internalization and recycling of caveolae.
Caveolae can flatten in response to membrane stretch and this mechanosensitive response of caveolae is thought to prevent membrane rupture during stress in addition to activating protective downstream signaling responses [reviewed in [13]. CAV1 is phosphorylated at Tyr14 in response to several mechanical stimuli, including integrin activation, resulting in the recruitment of SRC kinase inhibitor CSK to mediate actin reorganization. In response to mechanical stress, phosphocaveolin 1 increases caveola biogenesis, thereby helping the cell cope with cell surface stress [14]. CAV1 has been shown to regulate Rho dependent actomyosin contraction and in stromal fibroblasts this facilitates local tumor cell invasion and metastasis [15], although the role of caveolins in cancer is still unclear. Cavins most likely have a tumor suppressor function. Mutations in caveolar proteins are also associated with other diseases like skeletal muscle dystrophy, lipodystrophy and cardiac abnormalities (reviewed in [16]).
References
- Kovtun O, Tillu VA, Ariotti N, Parton RG, and Collins BM. Cavin family proteins and the assembly of caveolae. J. Cell. Sci. 2015; 128(7):1269-78. [PMID: 25829513]
- Ludwig A, Howard G, Mendoza-Topaz C, Deerinck T, Mackey M, Sandin S, Ellisman MH, and Nichols BJ. Molecular composition and ultrastructure of the caveolar coat complex. PLoS Biol. 2013; 11(8):e1001640. [PMID: 24013648]
- Gambin Y, Ariotti N, McMahon K, Bastiani M, Sierecki E, Kovtun O, Polinkovsky ME, Magenau A, Jung W, Okano S, Zhou Y, Leneva N, Mureev S, Johnston W, Gaus K, Hancock JF, Collins BM, Alexandrov K, and Parton RG. Single-molecule analysis reveals self assembly and nanoscale segregation of two distinct cavin subcomplexes on caveolae. Elife 2013; 3:e01434. [PMID: 24473072]
- Thomsen P, Roepstorff K, Stahlhut M, and van Deurs B. Caveolae are highly immobile plasma membrane microdomains, which are not involved in constitutive endocytic trafficking. Mol. Biol. Cell 2002; 13(1):238-50. [PMID: 11809836]
- Boucrot E, Howes MT, Kirchhausen T, and Parton RG. Redistribution of caveolae during mitosis. J. Cell. Sci. 2011; 124(Pt 12):1965-72. [PMID: 21625007]
- Mayor S, Parton RG, and Donaldson JG. Clathrin-independent pathways of endocytosis. Cold Spring Harb Perspect Biol 2014; 6(6). [PMID: 24890511]
- Pelkmans L, Kartenbeck J, and Helenius A. Caveolar endocytosis of simian virus 40 reveals a new two-step vesicular-transport pathway to the ER. Nat. Cell Biol. 2001; 3(5):473-83. [PMID: 11331875]
- Hayer A, Stoeber M, Ritz D, Engel S, Meyer HH, and Helenius A. Caveolin-1 is ubiquitinated and targeted to intralumenal vesicles in endolysosomes for degradation. J. Cell Biol. 2010; 191(3):615-29. [PMID: 21041450]
- Henley JR, Krueger EW, Oswald BJ, and McNiven MA. Dynamin-mediated internalization of caveolae. J. Cell Biol. 1998; 141(1):85-99. [PMID: 9531550]
- Morén B, Shah C, Howes MT, Schieber NL, McMahon HT, Parton RG, Daumke O, and Lundmark R. EHD2 regulates caveolar dynamics via ATP-driven targeting and oligomerization. Mol. Biol. Cell 2012; 23(7):1316-29. [PMID: 22323287]
- Echarri A, Muriel O, Pavón DM, Azegrouz H, Escolar F, Terrón MC, Sanchez-Cabo F, Martínez F, Montoya MC, Llorca O, and Del Pozo MA. Caveolar domain organization and trafficking is regulated by Abl kinases and mDia1. J. Cell. Sci. 2012; 125(Pt 13):3097-113. [PMID: 22454521]
- Muriel O, Echarri A, Hellriegel C, Pavón DM, Beccari L, and Del Pozo MA. Phosphorylated filamin A regulates actin-linked caveolae dynamics. J. Cell. Sci. 2011; 124(Pt 16):2763-76. [PMID: 21807941]
- Parton RG, and del Pozo MA. Caveolae as plasma membrane sensors, protectors and organizers. Nat. Rev. Mol. Cell Biol. 2013; 14(2):98-112. [PMID: 23340574]
- Joshi B, Bastiani M, Strugnell SS, Boscher C, Parton RG, and Nabi IR. Phosphocaveolin-1 is a mechanotransducer that induces caveola biogenesis via Egr1 transcriptional regulation. J. Cell Biol. 2012; 199(3):425-35. [PMID: 23091071]
- Goetz JG, Minguet S, Navarro-Lérida I, Lazcano JJ, Samaniego R, Calvo E, Tello M, Osteso-Ibáñez T, Pellinen T, Echarri A, Cerezo A, Klein-Szanto AJP, Garcia R, Keely PJ, Sánchez-Mateos P, Cukierman E, and Del Pozo MA. Biomechanical remodeling of the microenvironment by stromal caveolin-1 favors tumor invasion and metastasis. Cell 2011; 146(1):148-63. [PMID: 21729786]
- Nassoy P, and Lamaze C. Stressing caveolae new role in cell mechanics. Trends Cell Biol. 2012; 22(7):381-9. [PMID: 22613354]


