Shedding Light on Local Microtubule Regulation of Focal Adhesions
A Closer Look at Focal Adhesion Disassembly
Researchers from the Bershadsky Lab at MBI utilized optogenetics to unlock the role of microtubules in regulating focal adhesion disassembly, an important step in cell migration. This study was published in The EMBO Journal; https://doi.org/10.1038/s44318-024-00114-4
Within our bodies, tissues serve as bustling arenas for cellular interactions. They host a whirlwind of activity as resident cells move to carry out diverse biological functions, such as sensing the properties of their surroundings, reorganizing damaged tissues, and launching immune responses at sites of infection. Their movement on an underlying substrate or matrix is alike us walking on a solid ground or surface: anchoring or gripping just enough to stabilise, before letting go to move ahead. Too little grip, the cells may detach from the substrate and undergo untimely death, but too much grip, they may get stuck on the substrate and lose functions. To achieve an optimal grip, cells rely on specialised internal structures called focal adhesions.
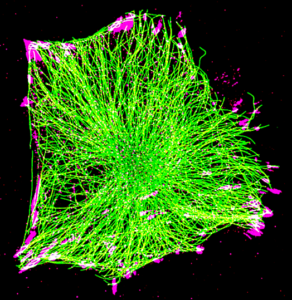
Focal adhesions are assemblies of proteins that connect to the cells’ cytoskeleton – composed of actin filaments (microfilaments), tubulin filaments (microtubules), and intermediate filaments. The cytoskeleton helps maintain cell shape, provide mechanical strength, and enable communication between cell components; and through their association with focal adhesions, they coordinate the behaviour of cells within a tissue.
Several cell-wide studies have highlighted the broader effects of microtubules on focal adhesion growth and turnover. More detailed research on how microtubule dynamics impact individual focal adhesions is needed to understand the key molecular events that control directed cell movement.
One such investigation was led by Dr. Julien Aureille, a research fellow at Prof. Alexander Bershadsky’s lab at the Mechanobiology Institute, National University of Singapore. Along with a collaborative team of researchers, Dr. Aureille used optogenetics – a technique that uses light to control cells – to develop a molecular tool that connects microtubules to specific focal adhesions upon blue light stimulation. Light was used to activate sensors found on two parts of a protein called KANK: one carried binding sites for focal adhesion components, and the other part carried binding sites for microtubule components. The two parts were physically linked upon light activation, which established a connection between the focal adhesions and microtubules. This allowed the researchers to directly examine how changes in microtubule dynamics affected those specific focal adhesions in real time.
Using the KANK constructs, the researchers manipulated the coupling and decoupling of microtubules and focal adhesions. They hypothesised a mechanistic model for how microtubules regulate the development and breakdown of focal adhesions. Two key molecules were involved: contractile actomyosin filaments and a signaling protein called GEF-H1.
When the KANK constructs were activated using blue light, microtubules gathered at specific focal adhesions before pulling away. The retraction triggered the accumulation of actomyosin filaments in the vicinity, creating traction forces, which made the focal adhesions slide and break apart.
Furthermore, the researchers discovered that GEF-H1 was released into the cell when the microtubules pulled back. GEF-H1 activates the Rho signaling pathway, increasing myosin filament assembly. When GEF-H1 was blocked, the accumulation of myosin filaments was prevented and subsequently, the focal adhesions did not slide or diassemble due to absence of tractional forces.
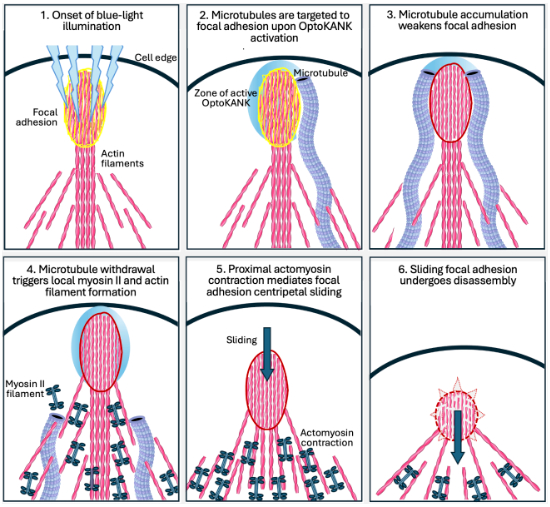
Progressive microtubule capture during focal adhesion maturation ultimately triggers focal adhesion turnover. Graphical abstract from https://doi.org/10.1038/s44318-024-00114-4
To validate their experimental findings, the team collaborated with Professor Alex Mogilner from New York University to develop a mathematical model of this novel microtubule-driven focal adhesion disassembly process. The model demonstrated consistency with the experimental data and provided a quantitative framework for understanding how microtubule targeting leads to the rapid disassembly of focal adhesions.
Cell migration is essential for physiological functions such as tissue organisation, wound healing and immune responses, and its disruption is implicated in diseases such as metastatic cancers. While the role of focal adhesions and its regulation by cytoskeletal components during cell migration is well-established, this study provides a detailed understanding of how microtubules interact with single focal adhesions.
By highlighting GEF-H1 and actomyosin filaments as crucial molecular players in focal adhesion turnover, the study also reveals potential targets that could be manipulated to regulate cell migration in health and disease. The team’s innovative use of optogenetics to manipulate cellular structures also presents a powerful approach that could help researchers further explore the complex interplay between different cellular components.