Defining the pattern of cell death and replacement
Maintenance of cell numbers in tissue requires mechanical forces to select cells to divide following programmed cell death
Researchers from the Toyama Lab at MBI uncover how mechanical factors including force and nuclear size direct cell death and replacement. This study was published in Developmental Cell. https://doi.org/10.1038/s44318-024-00114-4
Writer: Justin Lu. Editor: Andrew Wong.
When cells die through the naturally occurring process of apoptosis, or programmed cell death, the neighboring cells are stimulated to undergo cell division to fill the gap in the tissue left by the dead cell; this is a process known as compensatory proliferation, and it is essential to ensuring tissue homeostasis. This phenomenon was first discovered over 40 years ago when Dr. John Haynie and Prof. Peter Bryant conducted an experiment killing 60% of cells in Drosophila wing discs and observed that the remaining cells were remarkably able to restore the wing to its original size.
Research has shown that various mitogenic signals released by the apoptotic cell are shown to trigger cell proliferation in adjacent cells but interestingly, only certain adjacent cells are triggered. Thus, a long-standing mystery in the field of cell biology has been, “Why are only some neighboring cells triggered to divide after apoptosis?” A team of researchers from the Mechanobiology Institute and the Department of Biological Sciences, National University of Singapore, and contributors from Paris, France and Tokyo, Japan may have found the solution.
The team, led by MBI research fellows Takumi Kawaue, PhD and Ivan Yow, PhD, and graduate student Pan Yuping, all from the lab of Assoc. Prof Yusuke Toyama found that the non-uniform pattern of compensatory proliferation was linked to the non-uniform mechanosignalling of the Hippo-YAP pathway. Yes-associated protein (YAP) is a part of the Hippo signaling chain and is vital for apoptosis and proliferation regulation. To trigger cell division, YAP translocates to the nucleus. However, apart from this YAP nuclear translocation, they uncovered two additional factors are required to activate cell division: the cell must contain a large nucleus before apoptosis and experience significant stretching caused by mechanical force propagation during apoptosis.
Time-lapse images of MDCK YAP-GFP monolayer stained with Hoechst 33,342 (magenta). Asterisk, apoptotic cell; white arrowhead, YAP-GFP nuclear translocation; scale bars, 20 μm. https://doi.org/10.1016/j.devcel.2023.01.005
In order to reach this conclusion, the researchers grew cells on a substrate with fluorescent beads embedded on the surface, and targeted a UV laser to induce apoptosis on certain target cells. The researchers observed the movement of the beads to deepen their understanding of the forces taking place after apoptosis. As a result, they found that the substrate around the neighboring cells closest to the site of apoptosis experienced the most substrate deformation, as the fluorescent beads located in these areas were displaced the most.
Through further experimentation, they found that the cells within the extrusion site, where the apoptotic cell used to be, actually contracted rather than stretched. This contraction of the inner cells occurred 5-10 minutes after the outward movement of the surrounding cells. During apoptosis, an actomyosin cable forms within the dying cell and its neighbors, leading to cell extrusion and altering tissue tension. The time frame of inward movement was found to be aligned perfectly with the time of formation and contraction of these cables. This suggested that the substrate deformation was caused by the creation of these actomyosin cables and that it links to compensatory proliferation.
Analyzing the stretching of tissue, measured by bead displacement; nucleus size; and cell-cycle progression, measured by FUCCI (Fluorescent Ubiquitination-based Cell Cycle Indicator), which fascinatingly causes cells to change color according to its cell-cycle stage, the team found that following an apoptotic extrusion, only neighboring cells that had a large nucleus and experienced a high amount of strain were triggered to undergo cell division. Simultaneously, and expectedly, these cells were also the only cells in which the YAP translocated to the nucleus, triggering the Hippo signaling chain.
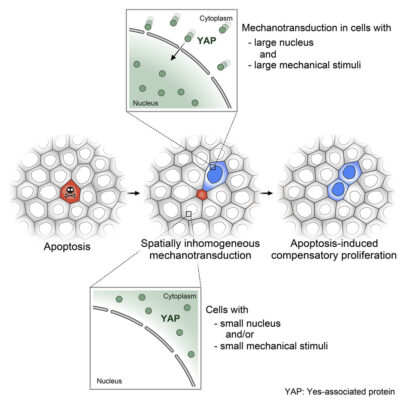
Graphical abstract illustration the role of mechanotransduction on apoptosis-induced compensatory proliferation. https://doi.org/10.1016/j.devcel.2023.01.005
As a result, the study found that after apoptosis, in order to ensure that cells do not overpopulate but also sufficiently replace the dead cells, apoptotic cells send signals that trigger division according to the intrinsic qualities of the cell as well as the amount of mechanical force propagation. Cells with larger nuclei that experienced greater mechanical strain were more likely to undergo YAP nuclear translocation and subsequent cell division. These findings suggest that while mitogenic signals from apoptotic cells are essential, the mechanical factors and intrinsic cellular properties also play crucial roles in determining which neighboring cells will proliferate.
Ivan Yow, Research Fellow: At the beginning, we observed the mechanical waves which fascinated us. However, frustration weighed heavily on me as I could not interpret this fascinating phenomenon and started doubting myself if this was all a mistake or caveat. It took years before I realized that what I thought was a mistake could be the doorway to a discovery I never imagined.
While there still remains much unknown about the phenomenon of compensatory proliferation in maintaining homeostasis, the MBI team have demonstrated that solving this biological mystery will require a fresh perspective that incorporates the role of mechanical force.